Class 12 Chemistry based on NCERT Syllabus
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electro chemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
Class 12 Chemistry Chapter 8 The d and f Block Elements:
| Section Name | Topic Name |
| 8 | The d – and f – Block Elements |
| 8.1 | Position in the Periodic Table |
| 8.2 | Electronic Configurations of the d-Block Elements |
| 8.3 | General Properties of the Transition Elements (d-Block) |
| 8.4 | Some Important Compounds of Transition Elements |
| 8.5 | The Lanthanoids |
| 8.6 | The Actinoids |
| 8.7 | Some Applications of d – and f -Block Elements |
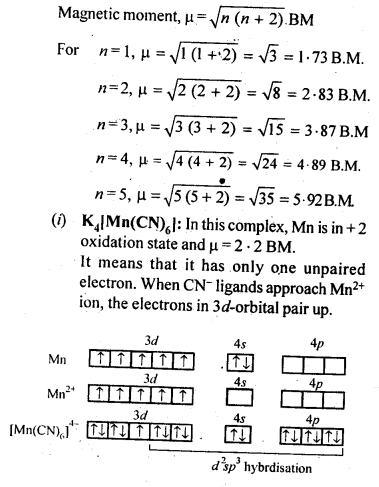
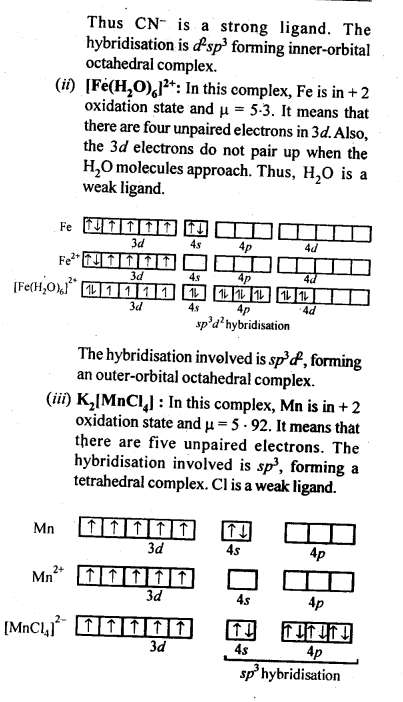
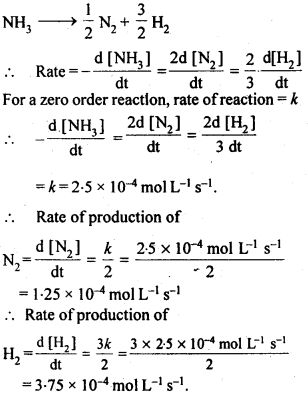
towards oxidation to their+3 state?
is more stable then Sc+ and Fe3+ is more stable than Fe2+ due to half filled it f-orbitals.
= + 5.
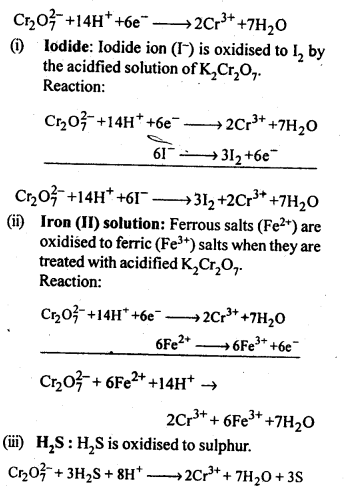
In the p-block the lower oxidation states are favoured by the heavier members (due to inert pair effect), the opposite is true in the groups of d-block. For example, in group 6, Mo(VI) and W(VI) are found to be more stable than Cr(VI). Thus Cr(VI) in the form of dichromate in acidic medium is a strong oxidising agent, whereas MoO3 and WO3 are not.
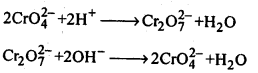
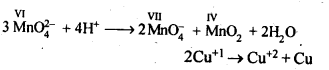
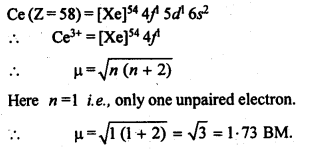

SCHOOL OF LEARNING COACHING AGARTALA
CRACK NEET JEE TBJEE WBJEE IIT NIT ENTRANCE
WITH SUBJECT EXPERTS & DOCTORATE, GOLD MEDALIST
LOWEST FEE IN INDIA
CLASS 12 TBSE CBSE NEET JEE ADMISSION 2024
LLB BBA CUET TPSC COACHING
ALL SUBJECTS IN ONE CLASSROOM
CLASS STARTS 1st April 2023
Learn with Gold Medalist, Doctorate, NITian, Asst Professor, AIR-1, Toppers Exp. Guide
What We Guide: School Of Learning Coaching
TBSE CBSE ICSE Board from Class VI-XII, College, University Students (Arts, Science and Commerce), Drawing, Music also available here.
SCHOOL OF LEARNING COACHING AGARTALA
CRACK NEET JEE TBJEE WBJEE IIT NIT ENTRANCE
WITH SUBJECT EXPERTS & DOCTORATE, GOLD MEDALIST
LOWEST FEE IN INDIA
We also guide Common University Entrance Test (CUET)- All India Level Entrance for the admission in Colleges and Universities.
*BA BSC LLB BBA BA-BEd BSC-BEd, IMD
Medical (NEET) and Engineering(JEE) Entrance,TPSC-Civil Services, Competitive Exams guidance also done by the experts.
Guidance for Teaching Job: Tripura TET & CTET, UGC NET/SLET
Competitive Govt Job Coaching
Vocational Training Course
- Medical & Engineering Entrance
Board + NEET + TBJEE
JEE (Main & Advanced)
- CUET (Common University Entrance Test)
Degree College Entrance
Eligibility: Class 12 Final Students
Master Degree Entrance
Eligibility: Degree 5-6th Semester Final Students
- College Tuition
B.A B.Sc B.Com (Pass & Honours) all subjects
- University Tuition
M.A. M.SC, M.COM
- TPSC-COMPETITIVE EXAMS
- COMPETITIVE EXAMS
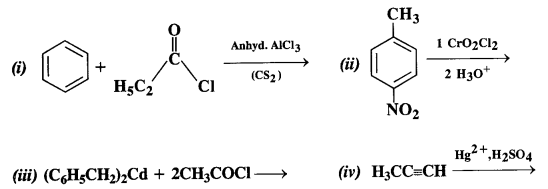
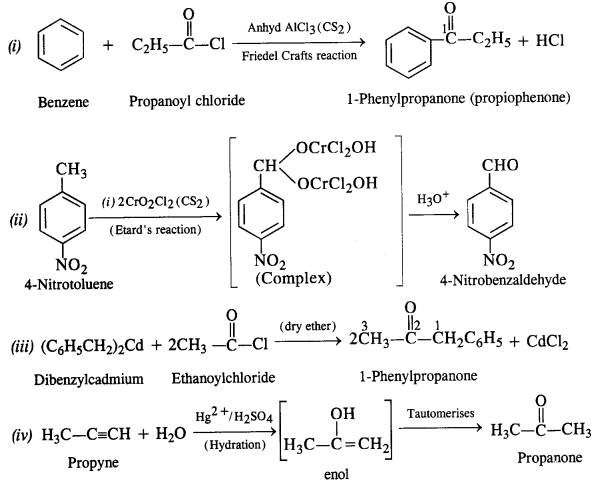
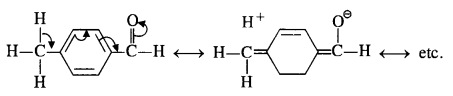
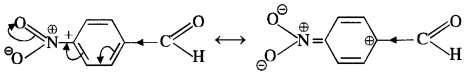
Chapter 12 Aldehydes Ketones and Carboxylic Acids
| Section Name | Topic Name |
| 12 | Aldehydes, Ketones and Carboxylic Acids |
| 12.1 | Nomenclature and Structure of Carbonyl Group |
| 12.2 | Preparation of Aldehydes and Ketones |
| 12.3 | Physical Properties |
| 12.4 | Chemical Reactions |
| 12.5 | Uses of Aldehydes and Ketones |
| 12.6 | Nomenclature and Structure of Carboxyl Group |
| 12.7 | Methods of Preparation of Carboxylic Acids |
| 12.8 | Physical Properties |
| 12.9 | Chemical Reactions |
| 12.10 | Uses of Carboxylic Acids |
NCERT INTEXT QUESTION
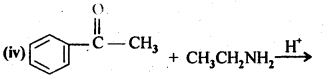
- No. of F atoms present in the molecule.
- Relative position of the F atom in the carbon atom chain.
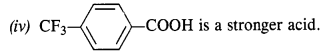
NCERT EXERCISES
(v) gem – Alkoxyalcohols are called hemiacetals. These are produced by addition of one molecule of a monohydric alcohol to an aldehyde in presence of dry HCl gas.
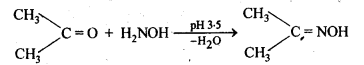
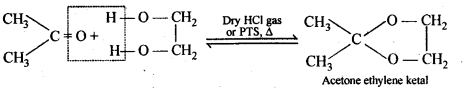


- decreases with increase in +I effect of the alkyl group.
-
decreases with increase in steric hindrance due to the size as well as number of the alkyl groups. In the light of the above information, the decreasing order of reactivity is :

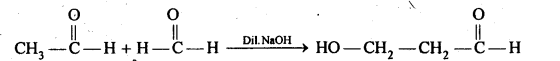



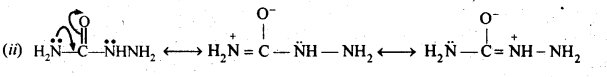
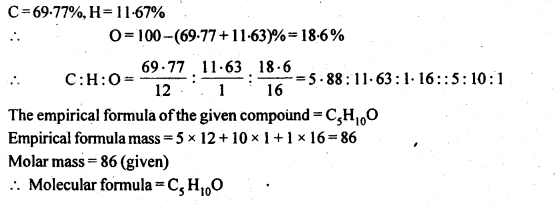
Class 12 Chemistry Chapter 3 Electrochemistry
| Section Name | Topic Name |
| 3 | Electrochemistry |
| 3.1 | Electrochemical Cells |
| 3.2 | Galvanic Cells |
| 3.3 | Nernst Equation |
| 3.4 | Conductance of Electrolytic Solutions |
| 3.5 | Electrolytic Cells and Electrolysis |
| 3.6 | Batteries |
| 3.7 | Fuel Cells |
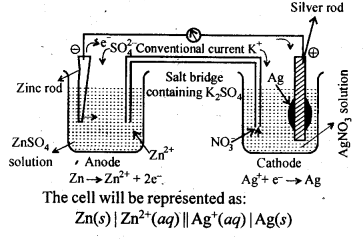
| 3.8 | Corrosion |
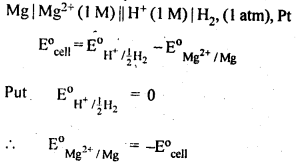
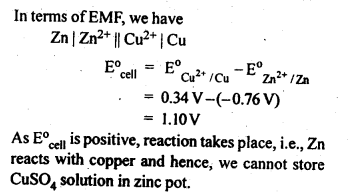
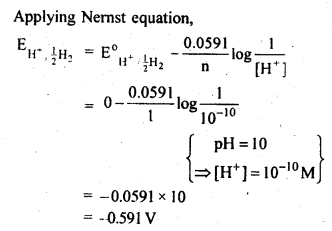
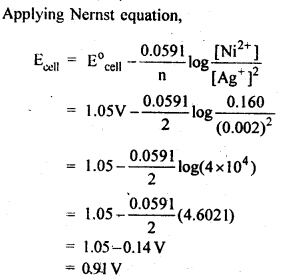
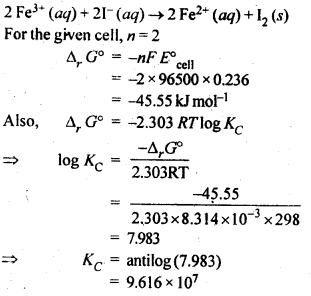

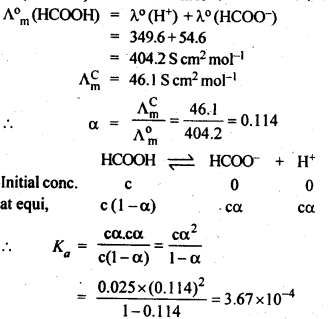
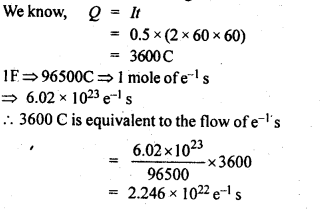
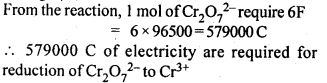
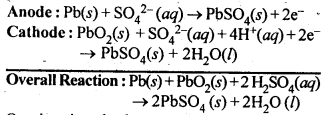
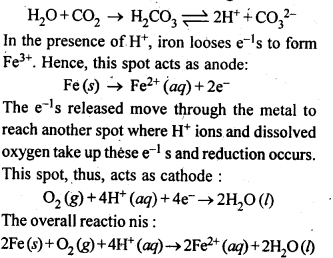
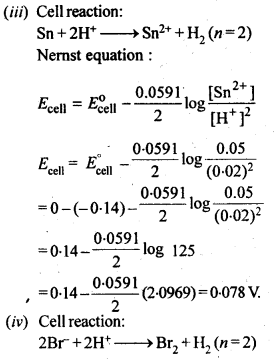
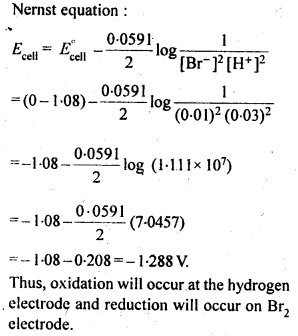
NCERT EXERCISES
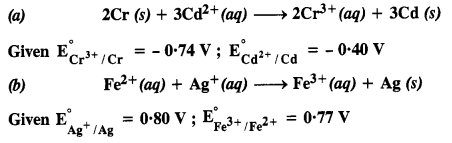
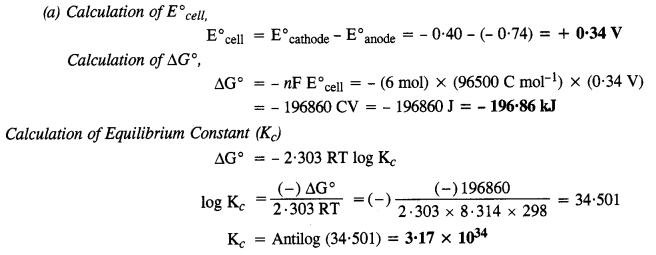
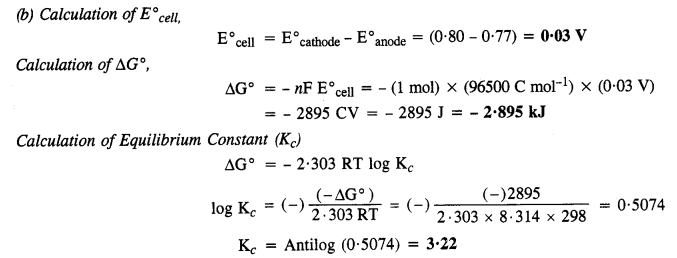
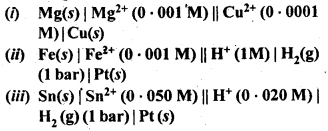
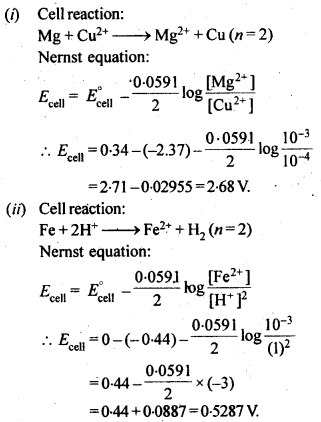
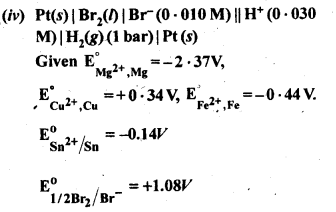
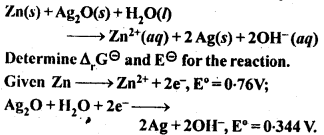
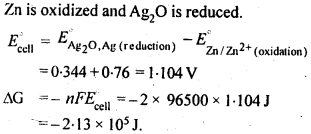
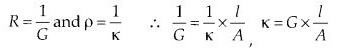
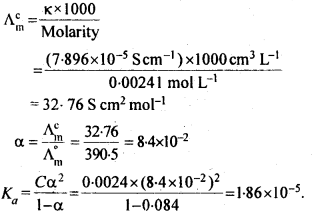
Hence, conductivity of a solution is defined as the conductance of a solution of 1 cm length and having 1 sq. cm as the area of cross-section. Alternatively, it may be defined as conductance of one centimetre cube of the solution of the electrolyte.
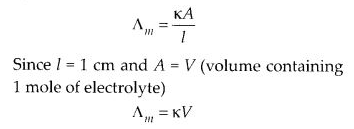
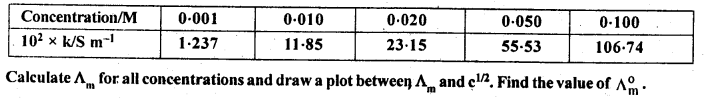
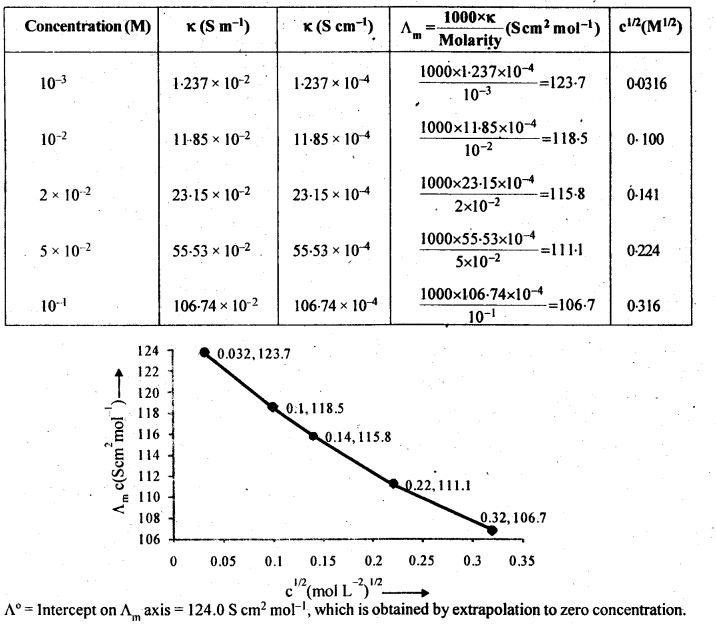
Molar conductivity increases with decrease in concentration. This is because that total volume, V, of solution containing one mole of electrolyte also increases. It has been found that decrease in K on dilution of a solution is more than compensated by increase in its volume.
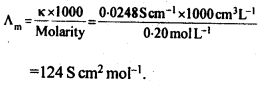
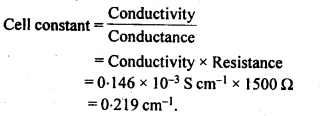
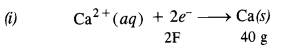
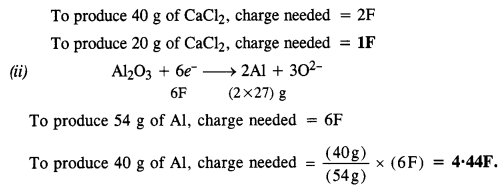
Chapter 1 The Solid State
Section Name
Topic Name
1
The Solid State
1.1
General Characteristics of Solid State
1.2
Amorphous and Crystalline Solids
1.3
Classification of Crystalline Solids
1.4
Crystal Lattices and Unit Cells
1.5
Number of Atoms in a Unit Cell
1.6
Close Packed Structures
1.7
Packing Efficiency
1.8
Calculations Involving Unit Cell Dimensions
1.9
Imperfections in Solids
1.10
Electrical Properties
1.11
Magnetic Properties
1.1. Why are solids rigid?
Ans: The constituent particles in solids have fixed positions and can oscillate about their mean positions. Hence, they are rigid.
1.2. Why do solids have definite volume?
Ans: Solids keep their volume because of rigidity in their structure. The interparticle forces are very strong. Moreover, the interparticle spaces are very few and small as well. As a result, their volumes cannot change by applying pressure.
1.3. Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fibreglass, copper
Ans: Crystalline solids: Benzoic acid, potassium nitrate, copper Amorphous solids: Polyurethane, Teflon, cellophane, polyvinyl chloride, fibreglass
1.4. Why is glass considered as super cooled liquid ? (C.B.S.E. Delhi 2013)
Ans: Glass is considered to be super cooled liquid because it shows some of the characteristics of liquids, though it is an amorphous solid. For example, it is slightly thicker at the bottom. This can be possible only if it has flown like liquid, though very slowly.
1.5. Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Ans: As the solid has same value of refractive index along all directions, it is isotropic in nature and hence amorphous. Being amorphous solid, it will not show a clean cleavage and when cut, it will break into pieces with irregular surfaces.
1.6. Classify the following solids in different categories based on the nature of the intermolecular forces: sodium sulphate, copper, benzene, urea, ammonia, water, zinc sulphide, diamond, rubedium, argon, silicon carbide.
Ans: Ionic, metallic, molecular, molecular, molecular (hydrogen-bonded), molecular (hydrogen-bonded), ionic, covalent, metallic, molecular, covalent (network).
1.7. Solid A is a very hard electrical insulator in. solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
Ans: It is a covalent or network solid.
1.8. Why are ionic solids conducting in the molten state and not in the solid-state?
Ans: In the ionic solids, the electrical conductivity is due to the movement of the ions. Since the ionic mobility is negligible in the solid state, these are non-conducting in this state. Upon melting, the ions present acquire some mobility. Therefore, the ionic solids become conducting
1.9. What type of solids are electrical conductors, malleable and ductile?
Ans: Metallic solids
1.10. Give the significance of a lattice point.
Ans: The lattice point denotes the position of a particular constituent in the crystal lattice. It may be atom, ion or a molecule. The arrangement of the lattice points in space is responsible for the shape of a particular crystalline solid.
1.11. Name the parameters that characterise a unit cell.
Ans: A unit cell is characterised by the following parameters:
(i)the dimensions of unit cell along three edges: a, b and c.
(ii)the angles between the edges: α (between b and c); β (between a and c) and γ (between a and b)
1.12. Distinguish between :
(i) Hexagonal and monoclinic unit cells
(ii) Face-centred and end-centred unit cells.
Ans:
(i) In a hexagonal unit cell :
a = b # c; α = β = 90° and γ = 120°
In a monoclinic unit cell :
a # b # c and α = γ = 90° and β # 90°
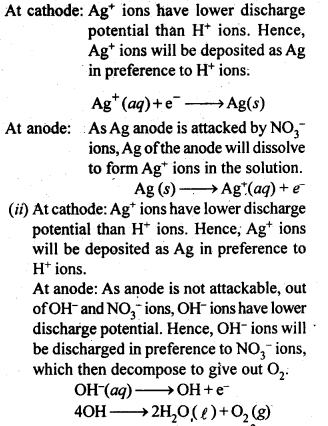
(ii) In a face-centered unit cell, constituent particles are located at all the corners as well as at the centres of all the faces.
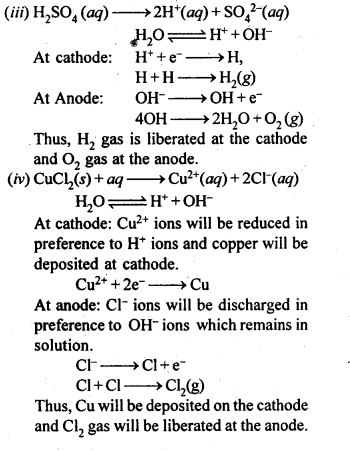
In end-centered unit cell, constituent particles are located at all the corners as well as at the centres of two opposite faces. (C.B.S.E Foreign 2015)
1.13. Explain how many portions of an atom located at
(i)corner and (ii)body centre of a cubic unit cell is part of its neighbouring unit cell.
Ans: (i) An atom at the comer is shared by eight adjacent unit cells. Hence, portion of the atom at the comer that belongs to one unit cell=1/8.
(ii)An atom at the body centre is not shared by any other unit cell. Hence, it belongs fully to unit cell.
1.14. What is the two-dimensional coordination number of a molecule in a square close-packed layer?
Ans: In the two-dimensional square close-packed layer, a particular molecule is in contact with four molecules. Hence, the coordination number of the molecule is four.
1.15. A compound forms hexagonal close-packed. structure. What is the total number of voids in 0. 5 mol of it? How many of these are tetrahedral voids?
Ans:
No. of atoms in close packings 0.5 mol =0.5 x 6.022 x 1023 =3.011 x 1023
No. of octahedral voids = No. of atoms in packing =3.011 x 1023
No. of tetrahedral voids = 2 x No. of atoms in packing
= 2 x 3.011 x 1023 = 6.022 x 1023
Total no. of voids = 3.011 x 1023 + 6.022 x 1023
= 9.033 x 1023
1.16. A compound is formed by two elements M and N. The element N forms ccp and atoms of the element M occupy 1/3 of the tetrahedral voids. What is the formula of the compound? (C.B.S.E. Foreign 2015)
Ans: Let us suppose that,
the no. of atoms of N present in ccp = x
Since 1/3rd of the tetrahedral voids are occupied by the atoms of M, therefore,
the no. of tetrahedral voids occupied = 2x/3
The ratio of atoms of N and M in the compound = x : 2x/3 or 3 : 2
∴ The formula of the compound = N3M2 or M2N3
1.17. Wh ich of the following lattices has the highest packing efficiency (i) simple cubic (ii) body-centered cubic and (iii) hexagonal close-packed lattice?
Ans: Packing efficiency of:
Simple cubic = 52.4% bcc = 68% hcp = 74%
hcp lattice has the highest packing efficiency.
1.18. An element with molar mass 2:7 x 10-2 kg mol-1 forms a cubic unit cell with edge length 405 pm. If its density is 2:7 x 103 kg m-3, what is the nature of the cubic unit cell ? (C.B.S.E. Delhi 2015)
Ans:
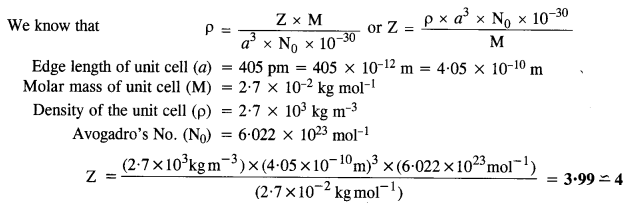 Since there are four atoms per unit cell, the cubic unit cell must be face centred (fcc) or cubic close packed (ccp).
Since there are four atoms per unit cell, the cubic unit cell must be face centred (fcc) or cubic close packed (ccp).
1.19. What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Ans: When a solid is heated, vacancy defect is produced in the crystal. On heating, some atoms or ions leave the lattice site completely, i.e., lattice sites become vacant. As a result-of this defect, density of the substances decreases.
1.20. What types of stoichiometric defects are shown by (C.B.S.E. Delhi 2013)
(i) ZnS
(ii) AgBr?
Ans:
(i) ZnS crystals may show Frenkel defects since the cationic size is smaller as compared to anionic size.
(ii) AgBr crystals may show both Frenkel and Schottky defects.
1.21. Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
Ans: Let us take an example NaCl doped with SrCl, impurity when SrCl2 is added to NaCl solid as an impurity, two Na+ ions will be replaced and one of their sites will be occupied by Sr21- while the other will remain vacant. Thus, we can say that when a cation of higher valence is added as an impurity to an ionic solid, two or more cations of lower valency are replaced by a cation of higher valency to maintain electrical neutrality. Hence, some cationic vacancies are created.
1.22. Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
Ans: Let us take an example of NaCl. When NaCl crystal is heated in presence of Na vapour, some Cl–ions leave their lattice sites to combine with Na to form NaCl. The e-1 s lost by Na to form Na+ (Na+ + Cl–—> NaCl) then diffuse into the crystal to occupy the anion vacancies. These sites are called F-centres. These e-s absorb energy from visible light, get excited to higher energy level and when they fall back to ground state, they impart yellow colour to NaCl crystal.
1.23. A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Ans: Impurity from group 15 should be added to get n-type semiconductor.
1.24. What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your answer.
Ans: Ferromagnetic substances make better permanent magnets. This is because when placed in magnetic field, their domains get oriented in the directions of magnetic field and a strong magnetic field is produced. This ordering of domains persists even when external magnetic field is removed. Hence, the ferromagnetic substance becomes a permanent magnet.
| Section Name | Topic Name |
| 1 | The Solid State |
| 1.1 | General Characteristics of Solid State |
| 1.2 | Amorphous and Crystalline Solids |
| 1.3 | Classification of Crystalline Solids |
| 1.4 | Crystal Lattices and Unit Cells |
| 1.5 | Number of Atoms in a Unit Cell |
| 1.6 | Close Packed Structures |
| 1.7 | Packing Efficiency |
| 1.8 | Calculations Involving Unit Cell Dimensions |
| 1.9 | Imperfections in Solids |
| 1.10 | Electrical Properties |
| 1.11 | Magnetic Properties |


1.1. Define the term ‘amorphous’. Give a few examples of amorphous solids.
Sol. Amorphous solids are those substances, in which there is no regular arrangement of its constituent particles, (i.e., ions, atoms or molecules). The arrangement of the constituting particles has only short-range order, i.e., a regular and periodically repeating pattern is observed over short distances only, e.g., glass, rubber, and plastics.
1.2. What makes glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Sol. Glass is a supercooled liquid and an amorphous substance. Quartz is the crystalline form of silica (SiO2) in which tetrahedral units SiO4 are linked with each other in such a way that the oxygen atom of one tetrahedron is shared with another Si atom. Quartz can be converted into glass by melting it and cooling the melt very rapidly. In the glass, SiO4 tetrahedra are joined in a random manner.
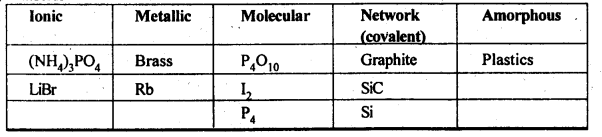
1.3 Classify each of the following solids as ionic, metallic, modular, network (covalent), or amorphous:
(i) Tetra phosphorus decoxide (P4O10) (ii) Ammonium phosphate, (NH4)3PO4 (iii) SiC (iv) I2 (v) P4 (vii) Graphite (viii), Brass (ix) Rb (x) LiBr (xi) Si
Sol.
1.4 (i) What is meant by the term ‘coordination number’?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body centred cubic structure?
Sol. (i) The number of nearest neighbours of a particle are called its coordination number.
(ii) (a) 12 (b) 8
1.5. How can you determine the atomic mass of an unknown metal if you know its density and dimensions of its unit cell ? Explain your answer. (C.B.S.E. Outside Delhi 2011)
Sol.
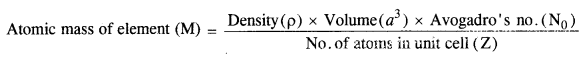
1.6 ‘Stability of a crystal is reflected in the magnitude of its melting points’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Sol. Higher the melting point, greater are the forces holding the constituent particles together and thus greater is the stability of a crystal. Melting points of given substances are following. Water = 273 K, Ethyl alcohol = 155.7 K, Diethylether = 156.8 K, Methane = 90.5 K.
The intermoleoilar forces present in case of water and ethyl alcohol are mainly due to the hydrogen bonding which is responsible for their high melting points. Hydrogen bonding is stronger in case of water than ethyl alcohol and hence water has higher melting point then ethyl alcohol. Dipole-dipole interactions are present in case of diethylether. The only forces present in case of methane is the weak van der Waal’s forces (or London dispersion forces).
1.7. How will you distinguish between the following pairs of terms :
(a) Hexagonal close packing and cubic close packing
(b) Crystal lattice and unit cell
(c) Tetrahedral void and octahedral void.
Sol.
(a) In hexagonal close packing (hcp), the spheres of the third layer are vertically above the spheres of the first layer
(ABABAB……. type). On the other hand, in cubic close packing (ccp), the spheres of the fourth layer are present above the spheres of the first layer (ABCABC…..type).
(b) Crystal lattice: It deplicts the actual shape as well as size of the constituent particles in the crystal. It is therefore, called space lattice or crystal lattice.
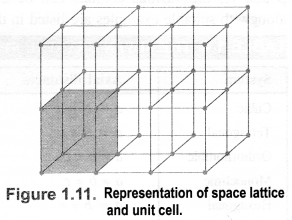 Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.
Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.
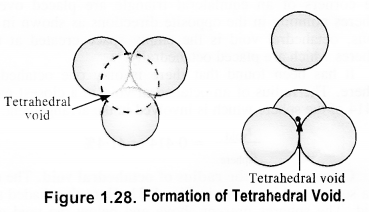
(c) Tetrahedral void: A tetrahedral void is formed when triangular void made by three spheres of a particular layer and touching each other.
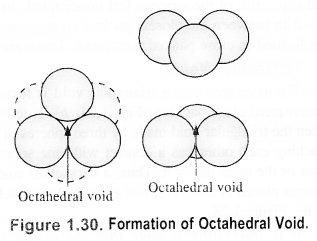
Octahedral void: An octahedral void or site is formed when three spheres arranged at the corners of an equilateral triangle are placed over anothet set of spheres.
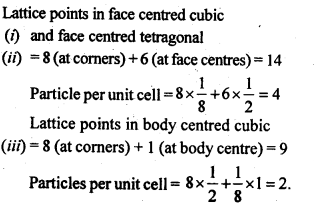
1.8 How many lattice points are there is one unit cell of each of the following lattices?
(i) Face centred cubic (if) Face centred tetragonal (iii) Body centred cubic
Sol.
1.9 Explain:
(i) The basis of similarities and differences between metallic and ionic crystals.
(ii) Ionic solids are hard and brittle.
Sol. (i) Metallic and ionic crystals
Similarities:
(a) There is electrostatic force of attraction in both metallic and ionic crystals.
(b) Both have high melting points.
(c) Bonds are non-directional in both the cases.
Differences:
(a) Ionic crystals are bad conductors of electricity in solids state as ions are not free to move. They can conduct electricity only in die molten state or in aqueous solution. Metallic crystals are good conductors of electricity in solid state as electrons are free to move.
(b) Ionic bond is strong due to strong electrostatic forces of attraction.
Metallic bond may be strong or weak depending upon the number of valence electrons and the size of the kernels.
(ii) Ionic solids are hard and brittle.Ionic solids are hard due to the presence of strong electrostatic forces of attraction. The brittleness in ionic crystals is due to the non- directional bonds in them.
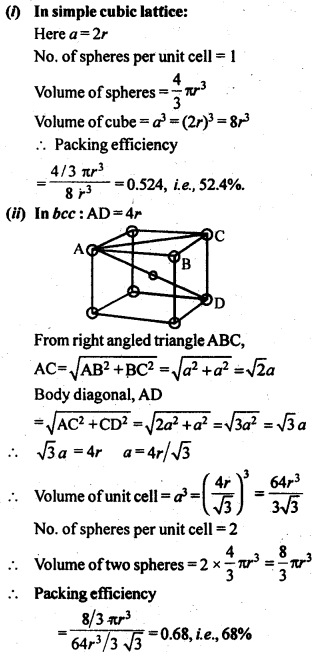
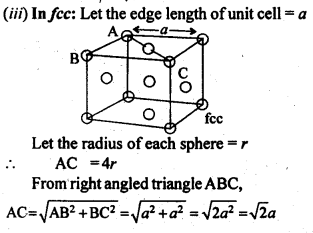
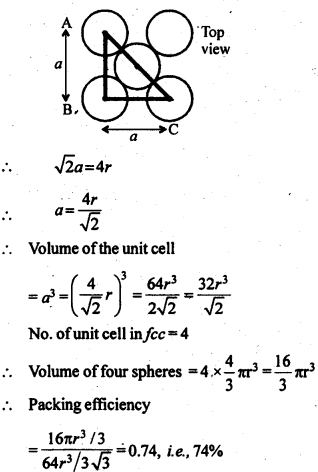
1.10 Calculate the efficiency of packing in case of a metal crystal for (i) simple cubic, (ii) body centred cubic, and (iii) face centred cubic (with the assumptions that atoms are touching each other).
Sol. Packing efficiency: It is the percentage of total space filled by the particles.
1.11 Silver crystallises in fcc lattice. If edge length of the cell is 4.07 x 10-8 cm and density is 10.5 g cm-3, calculate the atomic mass of silver.
Sol.
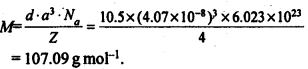
1.12. A cubic solid is made of two elements P and Q. Atoms Q are at the corners of the cube and P at the body centre. What is the formula of the compound ? What is the co-ordination number of P and Q?
Sol. Contribution by atoms Q present at the eight corners of the cube = 18 = x 8 = 1
Contribution by atom P present at the body centre = 1
Thus, P and Q are present in the ratio 1:1.
∴ Formula of the compound is PQ.
Co-ordination number of atoms P and Q = 8.
1.13 Niobium crystallises in a body centred cubic structure. If density is 8.55 g cm-3, calculate atomic radius of niobium, using its atomic mass 93u.
Sol.
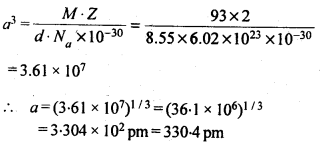
1.14 If the radius of the octahedral void is r and radius of the atoms in close-packing is R, derive relation between rand R.
Sol. A sphere is fitted into the octahedral void as shown in the diagram.
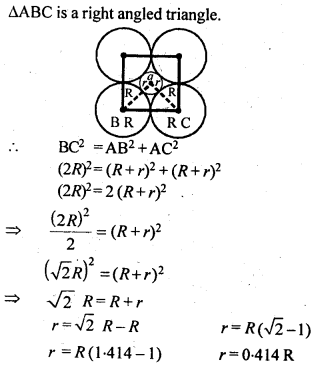
1.15 Copper crystallises into a fee lattice with edge length 3.61 x 10-8 cm. Show that the calculated density is in agreement with its measured value of 8.92 gcm-3.
Sol.
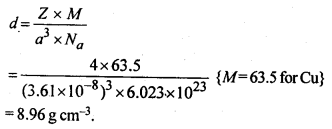 This calculated value of density is closely in agreement with its measured value of 8.92 g cm3.
This calculated value of density is closely in agreement with its measured value of 8.92 g cm3.
Question 16.
Analysis shows that nickel oxide has the formula Ni0.98 O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
Solution:
98 Ni-atoms are associated with 100 O – atoms. Out of 98 Ni-atoms, suppose Ni present as Ni2+ = x
Then Ni present as Ni3+ = 98 – x
Total charge on x Ni2+ and (98 – x) Ni3+ should
be equal to charge on 100 O2- ions.
Hence, x × 2 + (98 – x) × 3 = 100 × 2 or 2x + 294 – 3x = 200 or x = 94
∴ Fraction of Ni present as Ni2+ = 9498 × 100 = 96%
Fraction of Ni present as Ni3+ = 498 × 100 = 4%
Question 17.
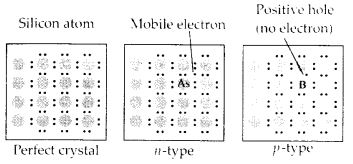
What are semi-conductors? Describe the two main types of semiconductors and contrast their conduction mechanisms.
Solution:
Semi-conductors are the substances whose conductivity lies in between those of conductors and insulators. The two
main types of semiconductors are n-type and p-type.
(i) n-type semiconductor: When a silicon or germanium crystal is doped with group 15 element like P or As, the dopant atom forms four covalent bonds like Si or Ge atom but the fifth electron, not used in bonding, becomes delocalised and continues its share towards electrical conduction. Thus silicon or germanium doped with P or As is called H-type semiconductor, a-indicative of negative since it is the electron that conducts electricity.
(ii) p-type semiconductor: When a silicon or germanium is doped with group 13 element like B or Al, the dopant is present only with three valence electrons. An electron vacancy or a hole is created at the place of missing fourth electron. Here, this hole moves throughout the crystal like a positive charge giving rise to electrical conductivity. Thus Si or Ge doped with B or Al is called p-type semiconductor, p stands for positive hole, since it is the positive hole that is responsible for conduction.
Question 18.
Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p-type semiconductor?
Solution:
The ratio less than 2 : 1 in Cu20 shows cuprous (Cu+) ions have been replaced by cupric (Cu2+) ions. For maintaining electrical neutrality, every two Cu+ ions will be replaced by one Cu2+ ion thereby creating a hole. As conduction will be due to the presence of these positive holes, hence it is a p-type semiconductor.
Question 19.
Ferric oxide crystallises in a hexagonal dose- packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Solution:
Suppose the number of oxide ions (O2-) in the packing = 90
∴ Number of octahedral voids = 90
As 2/3rd of the octahedral voids are occupied by ferric ions, therefore, number of ferric ions 2 present = 23 × 90 = 60
∴ Ratio of Fe3+ : O2- = 60 : 90 = 2 : 3
Hence, the formula of ferric oxide is Fe2O3.
Question 20.
Classify each of the following as being either a p-type or n-type semiconductor :
- Ge doped with In
- B doped with Si.
Solution:
- Ge is group 14 element and In is group 13 element. Hence, an electron deficient hole is created and therefore, it is a p – type semiconductor.
- B is group 13 element and Si is group 14 element, there will be a free electron, So, it is an n-type semiconductor.
Question 21.
Gold (atomic radius = 0.144 nm) crystallises in a face centred unit cell. What is the length of the side of the unit cell ?
Solution:
For a face centred cubic unit cell (fcc)
Edge length (a) = 22–√r = 2 x 1.4142 x 0.144 mm = 0.407 nm
Question 22.
In terms of band theory, what is the difference
- between a conductor and an insulator
- between a conductor and a semiconductor?
Solution:
In most of the solids and in many insulating solids conduction takes place due to migration of electrons under the influence of electric field. However, in ionic solids, it is the ions that are responsible for the conducting behaviour due to their movement.
(i) In metals, conductivity strongly depends upon the number of valence electrons available in an atom. The atomic orbitals of metal atoms form molecular orbitals which are so close in energy to each other, as to form a band. If this band is partially filled or it overlaps with the higher energy unoccupied conduction band, then electrons can flow easily under an applied electric field and the metal behaves as a conductor.
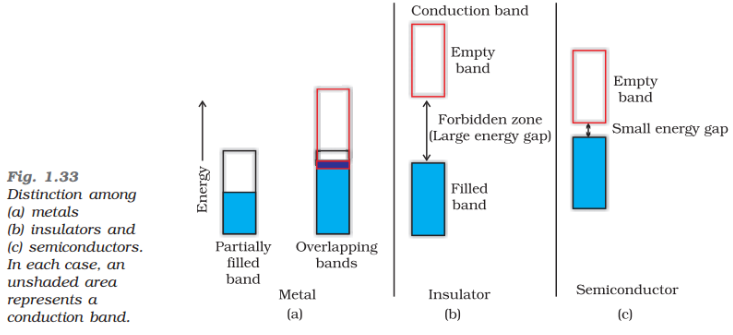
If the gap between valence band and next higher unoccupied conduction band is large, electrons cannot jump into it and such a substance behaves as insulator.
(ii) If the gap between the valence band and conduction band is small, some electrons may jump from valence band to the conduction band. Such a substance shows some conductivity and it behaves as a semiconductor. Electrical conductivity of semiconductors increases with increase in temperature, since more electrons can jump to the conduction band. Silicon and germanium show this type of behaviour and are called intrinsic semiconductors. Conductors have no forbidden band.
Question 23.
Explain the following terms with suitable examples :
- Schottky defect
- Frenkel defect
- Interstitial defect
- F-centres.
Solution:
(i) Schottky defect : In Schottky defect a pair of vacancies or holes exist in the crystal lattice due to the absence of equal number of cations and anions from their lattice points. It is a common defect in ionic compounds of high coordination number where both cations and anions are of the same size, e.g., KCl, NaCl, KBr, etc. Due to this defect density of crystal decreases and it begins to conduct electricity to a smaller extent.
(ii) Frenkel defect : This defect arises when some of the ions in the lattice occupy interstitial sites leaving lattice sites vacant. This defect is generally found in ionic crystals where anion is much larger in size than the cation, e.g., AgBr, ZnS, etc. Due to this defect density does not change, electrical conductivity increases to a small extent and there is no change in overall chemical composition of the crystal.
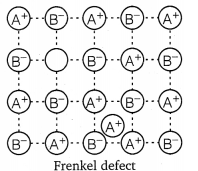
(iii) Interstitial defect : When some constituent particles (atoms or molecules) occupy an interstitial site of the crystal, it is said to have interstitial defect. Due to this defect the density of the substance increases.
(iv) F-Centres : These are the anionic sites occupied by unpaired electrons. F-centres impart colour to crystals. The colour results by the excitation of electrons when they absorb energy from the visible light falling on the crystal.
Question 24.
Aluminium crystallises in a cubic close packed structure. Its metallic radius is 125 pm.
- What is the length of the side of the unit cell?
- How many unit cells are there in 1.00 cm3 of aluminium?
Solution:
(i) For an fee unit cell, r = a22√ (given, r = 125 pm)
a = 2√2 r = 2√2 × 125 pm
= 353.55 pm
≅354 pm
(ii) Volume of one unit cell = a3 = (354 pm)3
= 4.4 × 107 pm3
= 4.4 × 107 × 10-30cm3
= 4.4 × 10-23 cm3
Therefore, number of unit cells in 1.00 cm3 =
= 2.27 × 1022
Question 25.
If NaCI is doped with 10-3 mol % SrCl2, what is the concentration of cation vacancies?
Solution:
Let moles of NaCI = 100
∴ Moles of SrCl2 doped = 10-3
Each Sr2+ will replace two Na+ ions. To maintain electrical neutrality it occupies one position and thus creates one cation vacancy.
∴ Moles of cation vacancy in 100 moles NaCI = 10-3
Moles of cation vacancy in one mole
NaCI = 10-3 × 10-2 = 10-5
∴ Number of cation vacancies
= 10-5 × 6.022 × 1023 = 6.022 × 1018 mol-1
Question 26.
Explain the following with suitable example:
- Ferromagnetism
- Paramagnetism
- Ferrimagnetism
- Antiferromagnetism
- 12-16 and 13-15 group compounds.
Solution:
(i) Ferromagnetic substances : Substances which are attracted very strongly by a magnetic field are called ferromagnetic substances, e.g., Fe, Ni, Co and CrO2 show ferromagnetism. Such substances remain permanently magnetised, once they have been magnetised. This type of magnetic moments are due to unpaired electrons in the same direction.
 The ferromagnetic material, CrO2, is used to make magnetic tapes used for audio recording.
The ferromagnetic material, CrO2, is used to make magnetic tapes used for audio recording.
(ii) Paramagnetic substances : Substances which are weakly attracted by the external magnetic field are called paramagnetic substances. The property thus exhibited is called paramagnetism. They are magnetised in the same direction as that of the applied field. This property is shown by those substances whose atoms, ions or molecules contain unpaired electrons, e.g., O2, Cu2+, Fe3+, etc. These substances, however, lose their magnetism in the absence of the magnetic field.
(iii) Ferrimagnetic substances : Substances which are expected to possess large magnetism on the basis of the unpaired electrons but actually have small net magnetic moment are called ferrimagnetic substances, e.g., Fe3O4, ferrites of the formula M2+Fe2O4 where M = Mg, Cu, Zn, etc. Ferrimagnetism arises due to the unequal number of magnetic moments in opposite direction resulting in some net magnetic moment.

(iv) Antiferromagnetic substances : Substances which are expected to possess paramagnetism or ferromagnetism on the basis of unpaired electrons but actually they possess zero net magnetic moment are called antiferromagnetic substances, e.g., MnO. Antiferromagnetism is due to the presence of equal number of magnetic moments in the opposite directions
(v) 13-15 group compounds : When the solid state materials are produced by combination of elements of groups 13 and 15, the compounds thus obtained are called 13-15 compounds. For example, InSb, AlP, GaAs, etc.
12-16 group compounds : Combination of elements of groups 12 and 16 yield some solid compounds which are referred to as 12-16 compounds. For example, ZnS, CdS, CdSe, HgTe, etc. In these compounds, the bonds have ionic character.

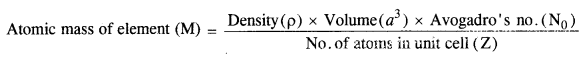
 Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.
Unit cell: Each bricks represents the unit cell while the block is similar to the space or crystal lattice. Thus, a unit cell is the fundamental building block of the space lattice.






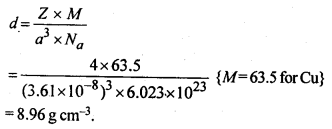

- Ge doped with In
- B doped with Si.
Solution:
- Ge is group 14 element and In is group 13 element. Hence, an electron deficient hole is created and therefore, it is a p – type semiconductor.
- B is group 13 element and Si is group 14 element, there will be a free electron, So, it is an n-type semiconductor.
- between a conductor and an insulator
- between a conductor and a semiconductor?

If the gap between valence band and next higher unoccupied conduction band is large, electrons cannot jump into it and such a substance behaves as insulator.
(ii) If the gap between the valence band and conduction band is small, some electrons may jump from valence band to the conduction band. Such a substance shows some conductivity and it behaves as a semiconductor. Electrical conductivity of semiconductors increases with increase in temperature, since more electrons can jump to the conduction band. Silicon and germanium show this type of behaviour and are called intrinsic semiconductors. Conductors have no forbidden band.
- Schottky defect
- Frenkel defect
- Interstitial defect
- F-centres.

(iii) Interstitial defect : When some constituent particles (atoms or molecules) occupy an interstitial site of the crystal, it is said to have interstitial defect. Due to this defect the density of the substance increases.
(iv) F-Centres : These are the anionic sites occupied by unpaired electrons. F-centres impart colour to crystals. The colour results by the excitation of electrons when they absorb energy from the visible light falling on the crystal.
- What is the length of the side of the unit cell?
- How many unit cells are there in 1.00 cm3 of aluminium?
- Ferromagnetism
- Paramagnetism
- Ferrimagnetism
- Antiferromagnetism
- 12-16 and 13-15 group compounds.

(ii) Paramagnetic substances : Substances which are weakly attracted by the external magnetic field are called paramagnetic substances. The property thus exhibited is called paramagnetism. They are magnetised in the same direction as that of the applied field. This property is shown by those substances whose atoms, ions or molecules contain unpaired electrons, e.g., O2, Cu2+, Fe3+, etc. These substances, however, lose their magnetism in the absence of the magnetic field.

(iv) Antiferromagnetic substances : Substances which are expected to possess paramagnetism or ferromagnetism on the basis of unpaired electrons but actually they possess zero net magnetic moment are called antiferromagnetic substances, e.g., MnO. Antiferromagnetism is due to the presence of equal number of magnetic moments in the opposite directions
(v) 13-15 group compounds : When the solid state materials are produced by combination of elements of groups 13 and 15, the compounds thus obtained are called 13-15 compounds. For example, InSb, AlP, GaAs, etc.
12-16 group compounds : Combination of elements of groups 12 and 16 yield some solid compounds which are referred to as 12-16 compounds. For example, ZnS, CdS, CdSe, HgTe, etc. In these compounds, the bonds have ionic character.
Class 12 Chemistry Chapter 8 The d and f Block Elements
| Section Name | Topic Name |
| 8 | The d – and f – Block Elements |
| 8.1 | Position in the Periodic Table |
| 8.2 | Electronic Configurations of the d-Block Elements |
| 8.3 | General Properties of the Transition Elements (d-Block) |
| 8.4 | Some Important Compounds of Transition Elements |
| 8.5 | The Lanthanoids |
| 8.6 | The Actinoids |
| 8.7 | Some Applications of d – and f -Block Elements |

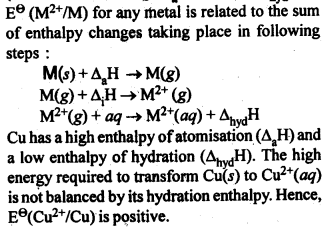

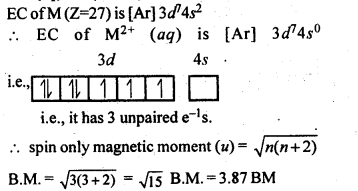
n(n+2)−−−−−−−
√
where n – number of unpaired electrons.
In the p-block the lower oxidation states are favoured by the heavier members (due to inert pair effect), the opposite is true in the groups of d-block. For example, in group 6, Mo(VI) and W(VI) are found to be more stable than Cr(VI). Thus Cr(VI) in the form of dichromate in acidic medium is a strong oxidising agent, whereas MoO3 and WO3 are not.
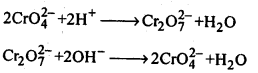


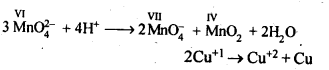

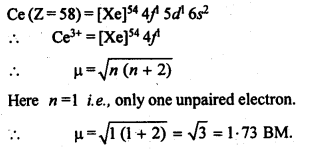




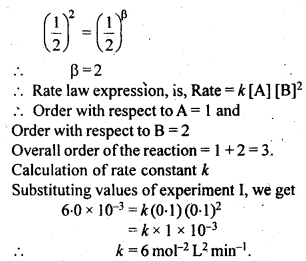
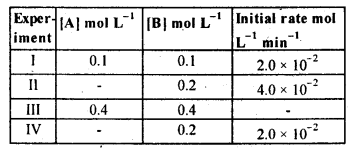
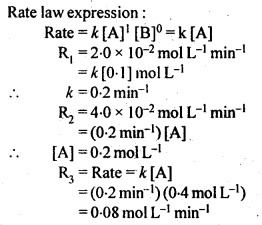
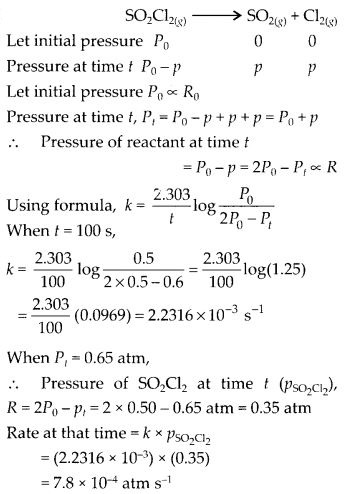
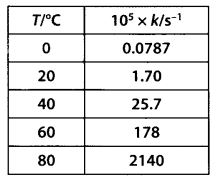
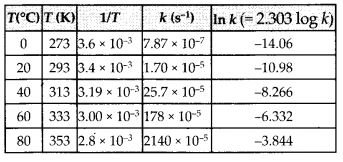
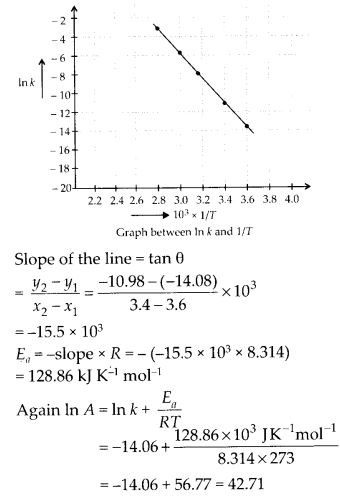
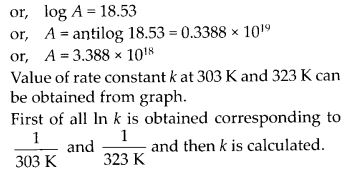
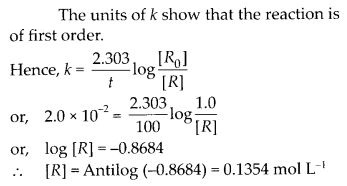
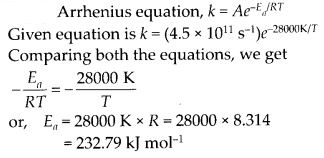
Class 12 Chemistry Chapter 4 Chemical Kinetics
| Section Name | Topic Name |
| 4 | Chemical Kinetics |
| 4.1 | Rate of a Chemical Reaction |
| 4.2 | Factors Influencing Rate of a Reaction |
| 4.3 | Integrated Rate Equations |
| 4.4 | Pseudo First Order Reaction |
| 4.5 | Temperature Dependence of the Rate of a Reaction |
| 4.6 | Collision Theory of Chemical Reactions |

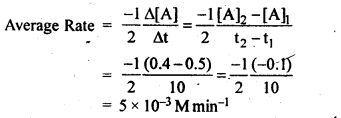
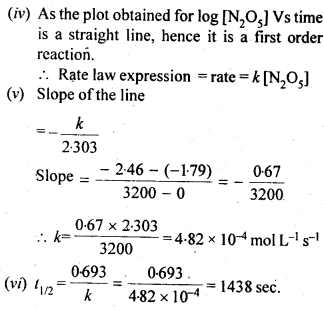
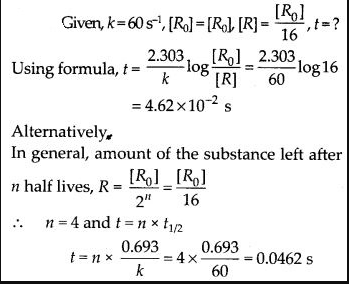
12
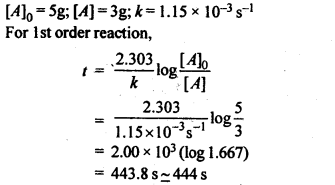
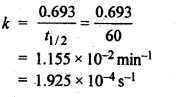
e
a
.
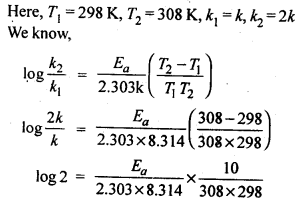
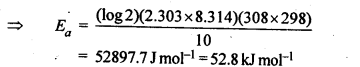
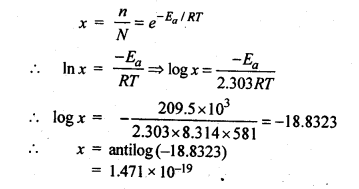
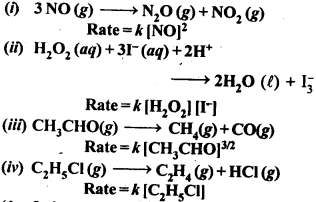
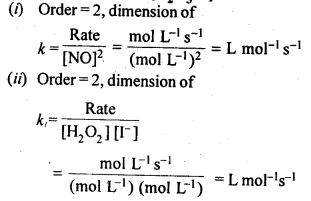
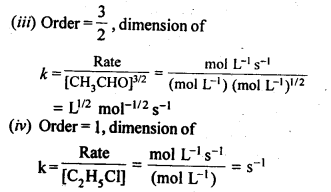
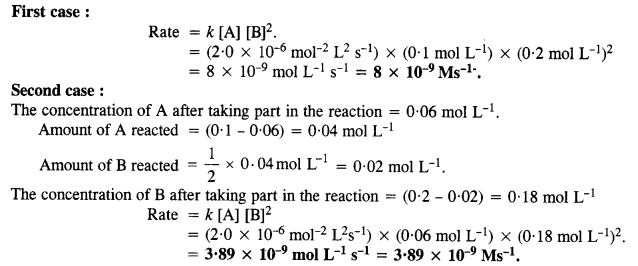
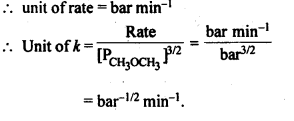
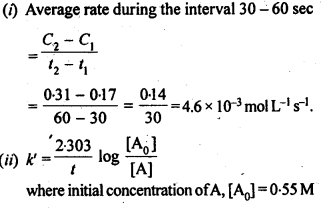
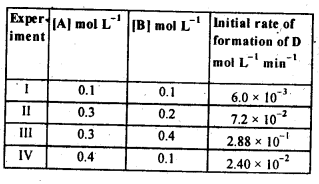
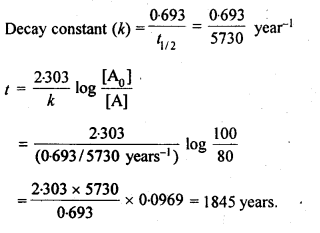
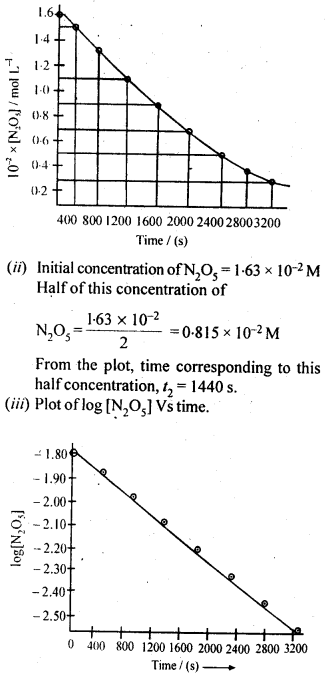
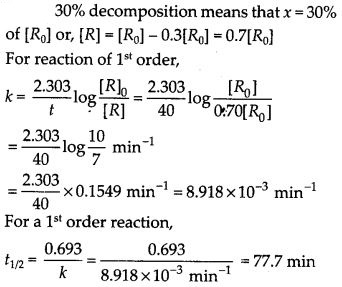
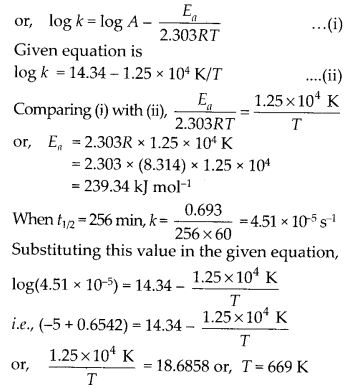
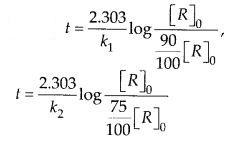
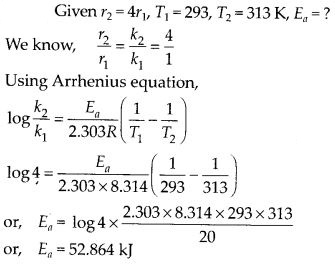
(ii) Temperature. In general, the increase in temperature increases the reaction rate (there are a few exceptions as well). Actually, the energy of the reactant species increases with the increase in temperature and so will be number of collisions. It has been observed that in most of the cases, about 10° increase in temperature makes reaction rate double. Please note that the effect of temperature is quite independent of the concentration of the reactant species.
(iii) Presence of catalyst. In many chemical reactions, the reaction rate can be enhanced by certain foreign substances called catalysts. These are actually not consumed in the reactions and also donot undergo any change in chemical characteristics. However, their physical states such as colour, particle size etc., might change. Certain catalysts may have adverse effect as well as the reaction rate. They result in decreasing the reaction rate instead of increasing it. These are called negative catalysts or inhibitors.

(v) Surface area. Increase in surface area provides more opportunity for the reactants to come in contact or collide resulting in increased reaction rate. For example, in laboratory. We quite often prefer granulated zinc lump of the metal while preparing hydrogen gas on reacting with dilute hydrochloric acid or dilute sulphuric acid. Actually, granulated zinc has greater surface area available for the attack by the acid than lump of zinc. Therefore, it reacts at a faster rate.
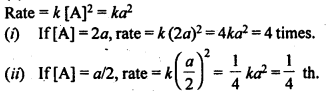
e
a
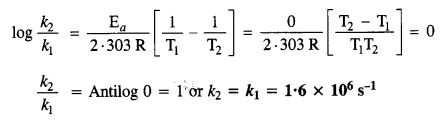
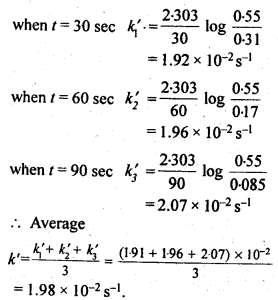


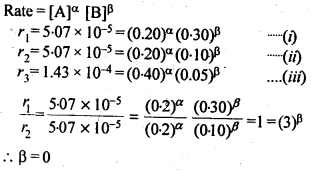

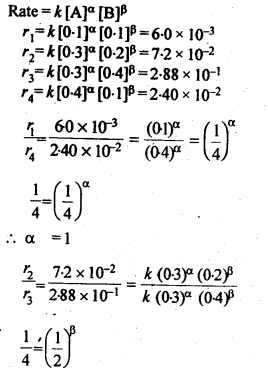
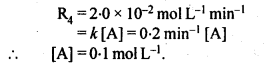


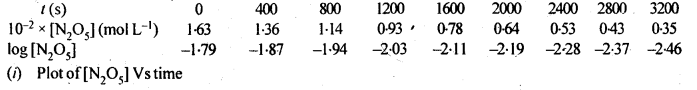

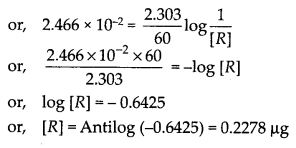
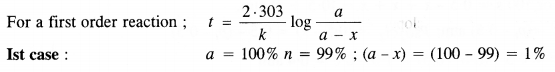
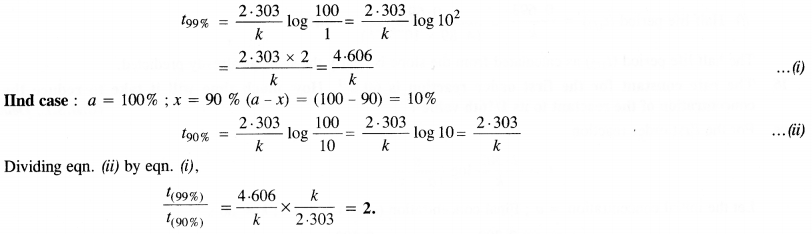

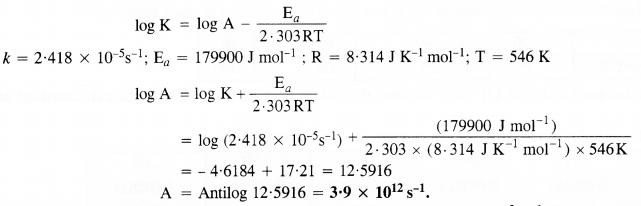





Class 12 Chemistry Chapter 5 Surface Chemistry
| Section Name | Topic Name |
| 5 | Surface Chemistry |
| 5.1 | Adsorption |
| 5.2 | Catalysis |
| 5.3 | Colloids |
| 5.4 | Classification of Colloids |
| 5.5 | Emulsions |
| 5.6 | Colloids Around Us |
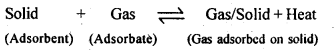
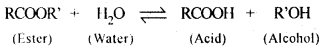
| Physisorption | Chemisorption |
| Weak van der Waals’ forces present. | Strong chemical bond forces present. |
| Low; of the order of 20-40 kJ/mol. | High; of the order of 80-240 kJ/mol. |
| Usually occurs at low temperature. | Occurs at high temperature. |
| Reversible. | Irreversible. |
| It is not specific in nature, i.e. all gases may be adsorbed on the surface of a solid. | It is highly specific in nature and occurs only when there is bond formation between adsorbent and adsorbate molecules. |
| Forms multi-molecular layers under high pressure. | Forms mono-molecular layer. |
| The extent of adsorption is directly related with the ease of liquefaction of the gas. | There is no correlation between extent of adsorption and the ease of liquefaction of the gas. |
| It does not involve appreciable activation energy. In most cases, the activation energy required is almost nil. | It generally requires appreciable activation energy since a chemical reaction is to take place. |
| Same state as in the bulk. | May be quite different from that in the bulk. |
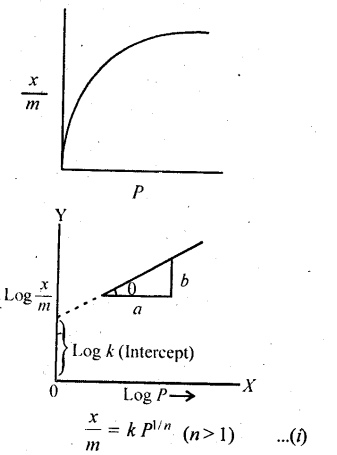
Nature of the adsorbate: The same gas is adsorbed to different extents by different solids at the same temperature. Also, greater the surface area of the adsorbent, more is the gas adsorbed.
Nature of the adsorbent: Different gases are adsorbed to different extents by different solids at the same temperature. Higher the critical temperature of the gas, greater is its amount adsorbed.
Surface area of the adsorbent: Surface area available for adsorption per gram of the adsorbent increases the extent of adsorption. Greater the surface area, higher would be the adsorption therefore, porous or powdered adsorbents are used.
Activation of adsorbent: It means increasing the adsorbing power of an adsorbent by increasing its surface area. It is done by :
- making the adsorbent’s surface rough
- removing gases already adsorbed
- subdividing the adsorbent into smaller pieces.
Pressure: At constant temperature, the adsorption of gas increases with pressure.
Temperature: Since adsorption is an exothermic process, applying Le Chatelier’s principle, we can find out that adsorption decreases with an increase in temperature.
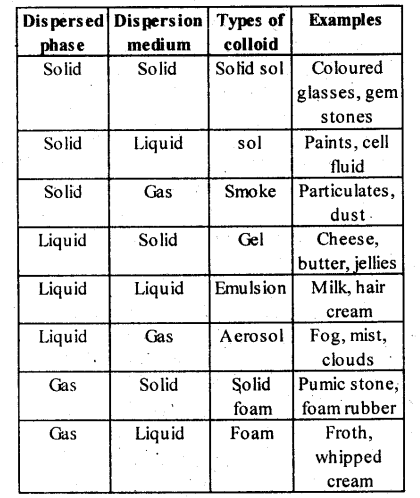
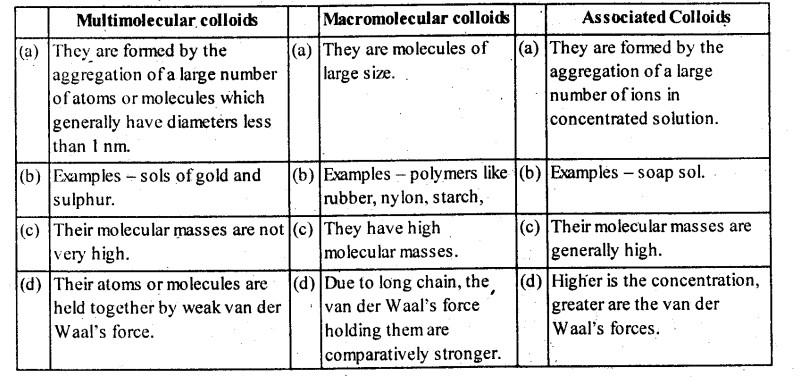
Lyophobic colloids: The colloidal solutions in which the particles of the dispersed phase have no affinity or love, rather have hatred for the dispersion medium, are called lyophobic colloids. The solutions of metals like Ag and Au, hydroxides like Al(OH)3 and Fe(OH)3 and metal sulphides like As2S3 are examples of lyophobic colloids.
Hydrocarbons
- Electrophoresis
- Coagulation
- Dialysis
- Tyndall effect
(ii) Coagulation or precipitation : The stability of the lyophobic sols is due to the presence of charge on colloidal particles. If somehow, the charge is removed, the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. The process of settling down of colloidal particles is called coagulation.
(iii) Dialysis : It is the process of removing dissolved substances from a colloidal solution by means of diffusion through a suitable membrane. Since particles (ions or smaller molecules) in a true solution can pass through animal membrane (bladder) or parchment paper or cellophane sheet but not the colloidal particles, the membrane can be used for dialysis. The apparatus used for this purpose is called dialyser. A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing. The molecules and ions diffuse through membrane into the outer water and pure colloidal solution is left behind.
(iv) Tyndall effect : Refer answer number 15 (i)
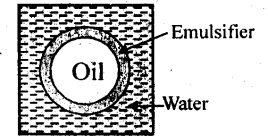
- Some of the medicines are effective as emulsions.
- Paints are emulsions which are used in our daily life.
- Soaps and detergents act as cleansing agents, action of which is based on emulsification.
- Photographic films are coated with emulsion of AgBr on its surface.
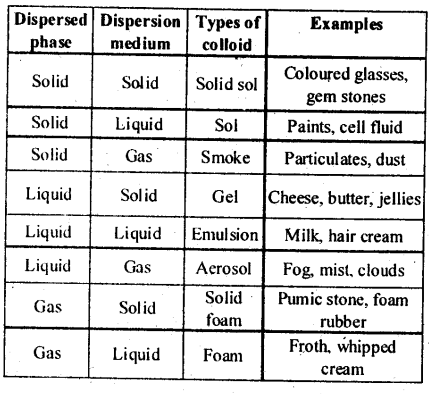
- Alcosol
- Aerosol
- Hydrosol
(ii) Aerosol : The sol in which dispersion medium is gas and dispersed phase is either solid or liquid, the colloidal system is called aerosol e.g., fog, insecticides, sprays, etc.
(iii) Hydrosol : The sol in which dispersion medium is water is called hydrosol e.g., starch sol.
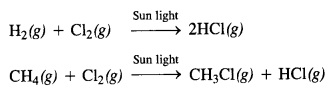
Class 12 Chemistry Chapter 7 The p Block Elements
| Section Name | Topic Name | Section Name | Topic Name |
| 7 | The p-Block Elements | 7.12 | Simple Oxides |
| 7.1 | Group 15 Elements | 7.13 | Ozone |
| 7.2 | Dinitrogen | 7.14 | Sulphur – Allotropic Forms |
| 7.3 | Ammonia | 7.15 | Sulphur Dioxide |
| 7.4 | Oxides of Nitrogen | 7.16 | Oxoacids of Sulphur |
| 7.5 | Nitric Acid | 7.17 | Sulphuric Acid |
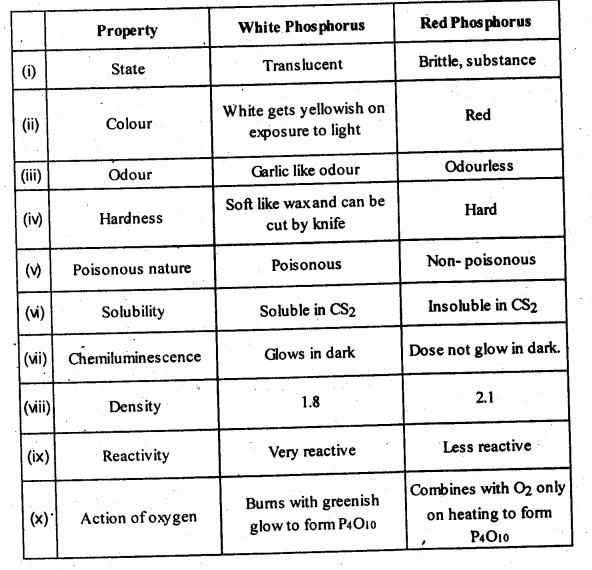
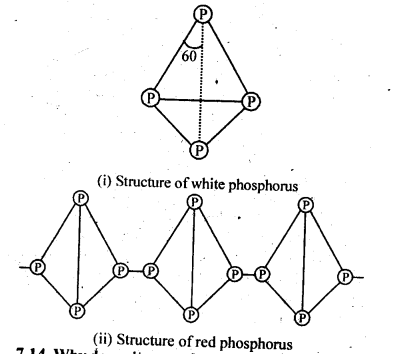
| 7.6 | Phosphorus – Allotropic Forms | 7.18 | Group 17 Elements |
| 7.7 | Phosphine | 7.19 | Chlorine |
| 7.8 | Phosphorus Halides | 7.20 | Hydrogen Chloride |
| 7.9 | Oxoacids of Phosphorus | 7.21 | Oxoacids of Halogens |
| 7.10 | Group 16 Elements | 7.22 | Interhalogen Compounds |
| 7.11 | Dioxygen | 7.23 | Group 18 Elements |
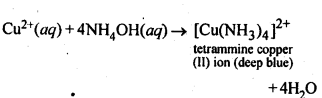
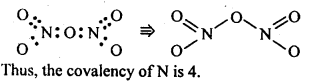
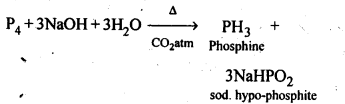
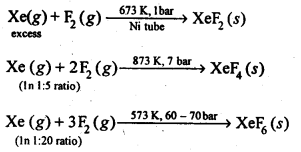
+4
ion higher than in PH3 ? (Pb. Board 2009)
+4
ion, the phosphorus atom is sp3 hybridised. However, in PH3 the central atom has apyramidal structure due to the presence of lone electron pair on the phosphorus atom.
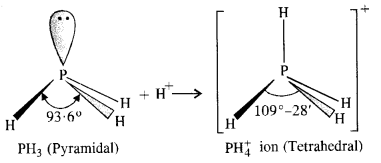
+4
ion, there is no lone electron pair on the phosphorus atom. It has a tetrahedral structure with bond angle of 109°-28′. Thus, the bond angle in
+4
ion is higher than in PH3.

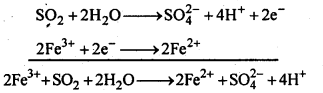
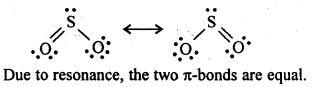
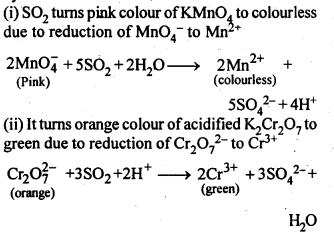
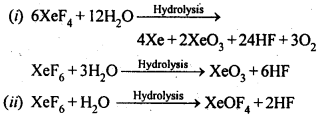
- Ionisation enthalpy, electro-negativity and electrode potential are higher for fluorine than the expected trends of other halogen.
- Fluorine does not show any positive oxidation state except in HOF.
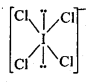
NCERT EXERCISES
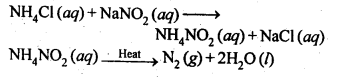
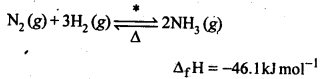
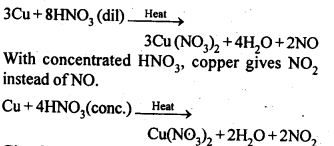
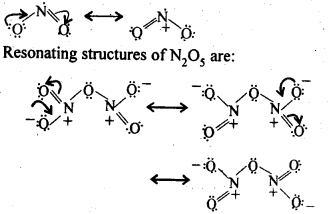
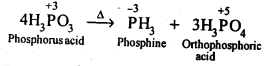
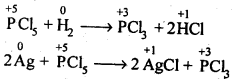

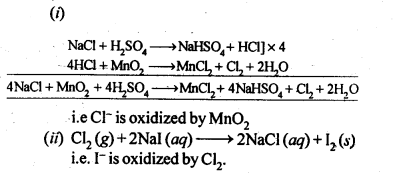
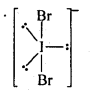
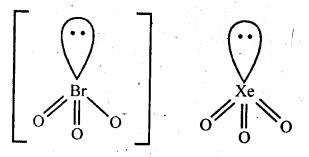
General Principles and Processes of Isolation of Elements
| Section Name | Topic Name |
| 6 | General Principles and Processes of Isolation of Elements |
| 6.1 | Occurrence of Metals |
| 6.2 | Concentration of Ores |
| 6.3 | Extraction of Crude Metal from Concentrated Ore |
| 6.4 | Thermodynamic Principles of Metallurgy |
| 6.5 | Electrochemical Principles of Metallurgy |
| 6.6 | Oxidation Reduction |
| 6.7 | Refining |
| 6.8 | Uses of Aluminium, Copper, Zinc and Iron |
6.1. Which of the ores mentioned can be concentrated by magnetic separation method?
Ans: Ores Which are magnetic in nature can be separated from non-magnetic gangue particles by magnetic separation method. For ex: ores of iron such as haemetite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) and iron pyrites (FeS2 ) being magnetic can be separated from non-magnetic silica and other impurities by magnetic separation method.
6.2. What is the significance of leaching in the extraction of aluminium?
Ans: Leaching or chemical separation is quite effective to purify bauxite an ore of aluminium associated with the impurities of iron oxide. The ore is leached with concentrated solution of NaOH to form a soluble complex leaving behind the impurities.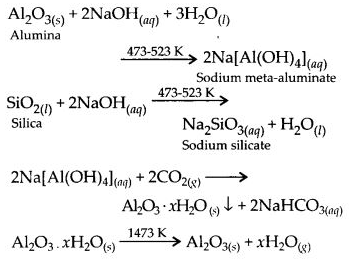
6.3.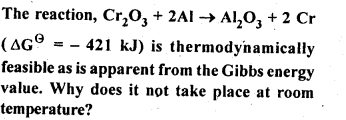
Ans: This is explained on the basis of Keq, the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no equilibrium between the reactants and products and hence the reactions does not occur at RT. At high temperature, Cr melts and values of TAS increases. As a result, the value of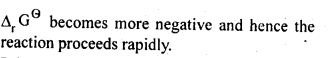
6.4. Is it true that under certain conditions, Mg can reduce Al203 and Al can reduce MgO? What are those conditions?
Ans: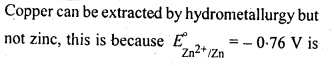
6.1. Copper can be extracted by hydrometallurgy but not zinc. Explain.
Ans: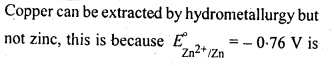
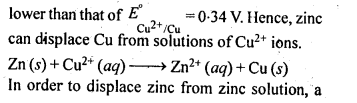
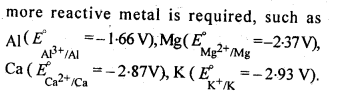
But with water, these metals (Al, Mg, Ca and K) forms their corresponding ions with the evolution of H2 gas.
Thus, Al, Mg, Ca, K, etc., cannot be used to displace zinc from zinc solution, and only copper can be extracted by hydrometallurgy but not the zinc.
6.2.What is the role of depressant in froth-floatation process?
Ans: The role of depressant is to prevent one type of sulphide ore particles from forming the froth with air bubbles. NaCN is used as a depressant to separate lead sulphide (PbS) ore from zinc sulphide (ZnS) ore. NaCN forms a zinc complex, Na2[Zn(CN)4] on the surface of ZnS thereby preventing it from the formation of the froth.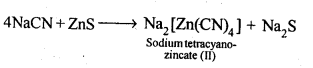
In this condition, only lead sulphide forms froth and thus can be separated from zinc sulphide ore.
6.3. Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Ans: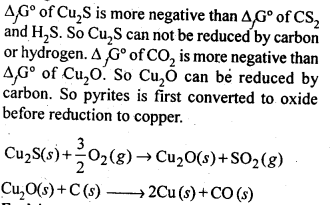
6.4. Explain:
(i)Zone refining
(ii)Column chromatography.
Ans: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., Ge, Si, B, Ca and In.
It is based on the principle that the impurities
are more soluble in the molten state (melt) than in the solid state of the metal.
The impure metal in the form of bar is heated at one end with a moving circular heater. As – the heater is slowly moved along the length of the rod, .the pure metal crystallises out of the melt whereas the impurities pass into the adjacent molten zone. Thi,s process is repeated several times till the impurities are completely driven to one end of the rod which is then cut off and discarded.
(ii) Chromatography: It is based on the principle that the different components of a mixture are adsorbed to different extents on an adsorbent.
In column chromatography, an adsorbent, such as alumina (Al2O3) or silica gel is packed in a column. This fonns the stationary phase. The mixture to be separated is dissolved in a suitable solvent (mobile phase) and applied to the top of the column. The adsorbed components are extracted (eluted) from the column with a suitable . solvent (eluent). The component which is more strongly adsorbed on the column takes longer time to travel through the column than a component which is weakly adsorbed. Thus, the various components of the mixture are seperated as they travel through absorbent (stationary phase).
6.5. Out of C and CO which is a better reducing agent at 673 K?
Ans: This can be explained thermodynamically, taking entropy and free energy changes into account.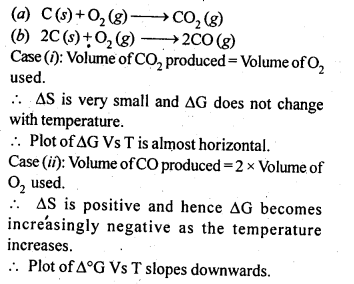
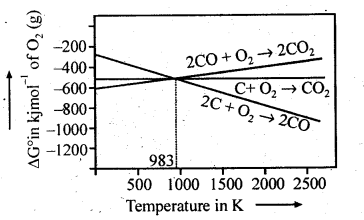
As can be seen from ΔG° Vs T plot (Ellingham diagram), lines for the reactions, C ——–> C02 and C ——–> CO cross at 983 K. Below 983 K, the reaction (a) is energetically more favourable but above 673 K, reaction (b) is favourable and preferred. Thus, below 673 K both C and CO can act as a reducing agent but since CO can be more easily oxidised to C02 than C to C02 , therefore, below 673 K, CO is more effective reducing agent than carbon.
6.6. Name the common elements present in anode mud in the electro-refining of copper. Why are they so present?
Ans: Anode mud contains metals like Ag, Au, Pt etc. which are less reactive than Cu. Actually, they are not in a position
to lose electrons though they constitute the electrode which acts as anode. All these metals are left as residue under anode (known as anode mud) while the entire copper present participates in the oxidation half reaction.
Cu(s) → Cu2+(aq) + 2e–
6.7. Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Ans: In the blast furnace reduction of iron oxides take place at different temperature ranges as shown below.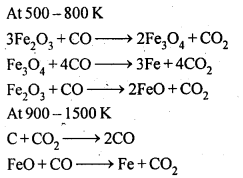
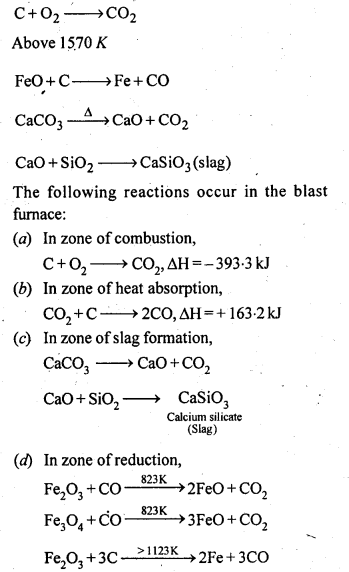
6.8. Write chemical reactions taking place in the extraction of zinc from zinc blende.
Ans: The following processes are involved in the extraction of zinc from zinc blende:
(i) Concentration: Zinc blende ore is crushed and the concentration done by froth- floatation process.
(ii) Roasting: The concentrated ore is then roasted in presence of excess of air at about 1200 K as a result zinc oxide is formed.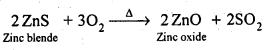
(iii) Reduction : Zinc oxide obtained above is mixed with powdered coke and heated to 1673 K in a fire clay retort where it is reduced ‘ to zinc metal.![]()
At 1673 K, zinc metal being volatile (boiling point 1180 K), distills over and is condensed.
(iv) Electrolytic refining: Impure zinc is made the anode while pure zinc strip is made the cathode. ZnSO4 solution acidified with dil. H2SO4 is the electrolyte used. On passing electric current, pure zinc gets deposited on the cathode.
6.9. State the role of silica in the metallurgy of copper.
Ans: Silica (SiO2) acts as an acidic flux in the metallurgy of copper and combines with FeO (the main impurity) to form FeSiO3 which is a slag.![]()
6.10. What is meant by the term “chromatography”?
Ans: Chromatography is a technique used for separation, purification, identification and characterization of the components of a mixture whether coloured or colourless. The term chromatography was originally derived from the Greek word ‘chroma’ meaning colour and ‘graphy for writing because the method was first used for the separation of coloured substances (plant pigments) into individual components.
6.11. What criterion is followed for the selection of the stationary phase in chromatography?
Ans: In chromatography, particularly in adsorption chromatography, the stationary phase is the adsorbent. It should fulfil certain criteria for better results.
(i) It should have high but selective adsorption power.
(ii) The particles should be spherical in shape and of uniform size.
(iii) The adsorbent should not react chemically with the solvents used for elution or with the components of the mixture under investigation.
(iv) The adsorbent should contain as small amount of the soluble components as possible.
(v) The adsorbent should be catalytically inactive and must have a neutral surface.
(vi) The adsorbent should be easily available.
(vi) The adsorbent should be perfectly white.
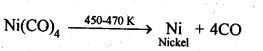
6.12. Describe a method for refining nickel.
Ans: When impure nickel is heated in presence of CO at 330-350 K, it forms volatile nickel tetracarbonyl leaving behind the impurities. The nickel tetracarbonyl thus obtained is then heated to higher temperature (450-470K), then it undergoes thermal decomposition to give pure nickel.![]()
6.13. How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
Ans: Pure alumina can be separated from silica in bauxite by Baeyer’s process. The bauxite ore associated with silica is heated with a concentrated solution of NaOH at 473-523 K and 35-36 bar pressure. Under these conditions, alumina dissolves as sodium meta-aluminate and silica as sodium silicate leaving behind the impurities.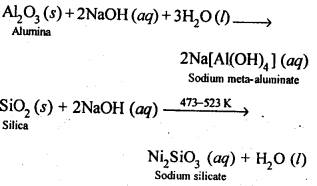
The resulting solution is filtered to remove the undissolved impurities, sodium meta-aluminate can be precipitated as hydrated aluminium oxide by passing CO2 vapours. The sodium silicate formed cannot be precipitated and can be filtered off.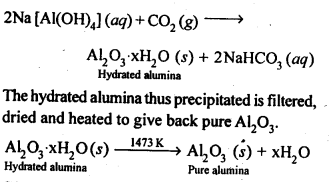
6.14. Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Ans: Calcination is a process of converting carbonates and hydroxide ores of metals to their respective oxides by heating them, strongly below their melting points either in absence or limited supply of air.![]()
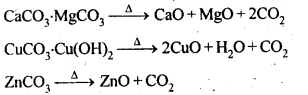
Roasting is a process of converting sulphide ores into its metallic oxides by heating strongly below its melting point in excess of air.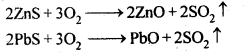
6.15. How is ‘cast-iron’ different from ‘pig iron’?
Ans: Cast iron differs from pig iron with respect to the carbon contents. Whereas carbon contents in pig iron are nearly four percent (4%), cast iron contains carbon to the extent of nearly three percent (3%).
6.16. Differentiate between “minerals” and “ores’.
Ans: Minerals: The natural substances in which the metals or their compounds occur in the earth is called minerais.
Ores: The minerals from which the metals can be coaveniently and economically extracted are called ores.
Note : All ores are minerals but all minerals are not ores.
6.17. Why copper matte is put in silica lined converter?
Ans: Copper matte consists of Cu2S along with some unchanged FeS. When a blast of hot air is passed through molten matte placed in silica lined converter, FeS present in matte is oxidised to FeO which combines with silica (SiO2) to form FeSiO3slag.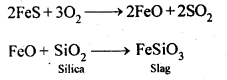 2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal.
2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal.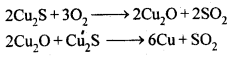
Thus, copper matte is heated in silica lined converter to remove FeS present in matte as FeSiO3 slag.
6.18. What is the role of cryolite in the metallurgy of aluminium?
Ans: (a) It lowers the fusion (melting) point of the bath from 2323 K to about 1140 K.
(b) It makes alumina a good conductor of electricity.
6.19. How is leaching carried out in case of low grade copper ores?
Ans: Leaching in case of low grade copper ores is carried out with acids in presence of air. In this process, copper is oxidised to Cu2+ ions which pass into the solution.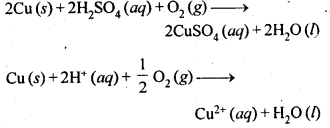
6.20. Why is zinc not extracted from zinc oxide through reduction using CO?
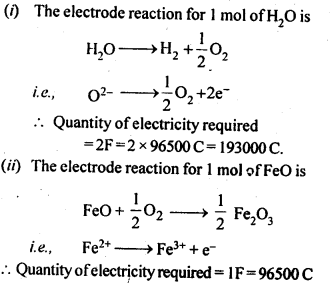
Ans: The chemical reaction involving the reduction of ZnO by CO is :
ZnO(s) + CO(g) → Zn(s) + CO2(g)
The process is thermodynamically not feasible because there is hardly any change in entropy as a result of the reaction. This is quite evident from the physical states of the reactants and products involved in the reaction
6.21. The value of ΔfG° for formation of Cr2O3 is – 540 kJ mol-1 and that of Al203 is – 827 kJ mol-1 . Is the reduction of Cr2O3 possible with Al?
Ans: Chemical equation for the formation of Cr2O3 and Al203 are as follows :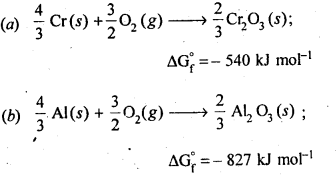
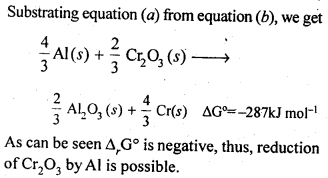
6.22. Out of C and CO, which is a better reducing agent for ZnO?
Ans: The two reduction reactions are :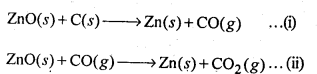
In the first case, there is increase in the magnitude of ΔS° while in the second case, it almost remains the same. In other words ΔG° will have more negative value in the first case when C(s) is used as the reducing agent than in the second case when CO(g) acts as the reducing agent. Therefore, C(s) is a better reducing agent.
6.23. The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Ans: We can study the choice of a reducing agent in a particular case using Ellingham diagram.
It is evident from the diagram that metals for which the standard free energy of formation oftheir oxides is more negative can reduce those metal oxides for which the standard free energy of formation of their respective oxides is less negative. It means that any metal will reduce the oxides of other metals which lie above it in the Ellingham diagram. This is because the standard free energy change (ΔrG°) of the combined redox reaction will be negative by an amount equal to the difference in Δf G° of the two metal oxides. Thus both Al and Zn can reduce FeO to Fe but Fe cannot reduce Al203 to A1 and ZnO to Zn. In the same way, G can reduce ZnO to Zn but not CO.
Note : Only that reagent will be preferred as reducing agent which will lead to decrease in free energy value (ΔG°) at a certain specific temperature.
6.24. Name the processes from which chlorine is obtained as a by-product What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Ans: Down process is used for the preparation of sodium metal, where chlorine is obtained as a by- product. This -process involves the electrolysis of a fused mixture of NaCl and CaCl2 at 873 K.Sodium is discharged at the cathode while Cl2 is obtained at the anode as a by-product.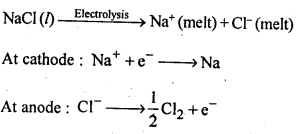
If, an aqueous solution of NaCl is electrolysed, H2 is evolved at the cathode while Cl2 is obtained at the anode.
6.25. What is the role of graphite rod in the electrometallurgy of aluminium?
Ans: In the electrometallurgy of aluminium, oxygen gas is evolved at anode. O2 reacts with graphite or carbon (graphite electrodes) to form carbon monoxide and carbon dioxide. In case if some other metal electrodes is used as anode, then oxygen will react with aluminium formed during the process to form aluminium oxide(Al2O3) which will pass into the reaction mixture resulting into wastage of Al. Since graphite is cheaper than aluminium, its wastage or can be tolerated.
6.26. Outline the principles of refining of metals by the following methods:
(i)Zone refining
(ii)Electrolytic refining
(iii)Vapour phase refining
Ans: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., Ge, Si, B, Ca and In.
It is, based on the principle that the impurities are more soluble in the molten state (melt) than in the solid state of the metal.
The impure metal in the form of bar is heated at one end with a moving circular heater. As the heater is slowly moved along the length of the rod, the pure metal crystallises out of the melt whereas the impurities pass into the adjacent molten zone. This process is repeated several times till the impurities are completely driven to one end of the rod which is then cut off and discarded.
(ii)Electrolytic refining: Many metals, such as Cu, Ag, Au, Al, Pb, etc., are purified by this method. The impure metals is made the anode while a thin sheet of pure metal acts as a cathode. The electrolytic solution consists of a salt or a complex salt solution of the metal. On passing the current, the pure metal is deposited on the cathode while the impurities fall down as anode mud.
(iii)Vapour-phase refining: The crude metal is freed from impurities by first converting it into a suitable volatile compound by heating it with a specific reagent at a lower temperature and then decomposing the volatile compound at some higher temperature to give the pure metal.
(a)Mond’s process: When impure nickel is heated is a current of CO at 330-350 K, it forms volatile nickel tetracarbonyl complex leaving behind the impurities. The complex then heated to a higher temperature (450-470K) when it undergoes thermal decomposition giving pure nickel.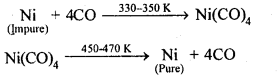
(b)Van Arkel method: This method is Used for preparing ultra-pure metals by removing all the oxygen and nitrogen present as impurities in metals like zirconium and titanium (which are used in space technology).Crude Zr is heated in an evacuated vessel with iodine at 870 K. Zirconium tetraiodide thus formed is separated. It is then decomposed by heating over a tungsten filament at 1800 – 2075 K to give pure Zr.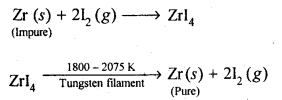
6.27. Predict conditions under which Al might be expected to reduce MgO.
Ans: The equations for the formation of the two oxides are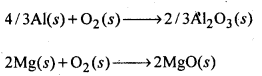
If we look at the plots for the formation of the two oxides of the Ellingham diagram, we find that they intersect at certain point. The corresponding value of ΔG° becomes zero for the reduction of MgO by Al metal.
![]()
This means that the reduction of MgO by Al metal can occur below this temperature. Aluminium (Al) metal can reduce MgO to Mg above this temperature because Δ°G for Al2O3 is less as compared to that of MgO.![]()
Chapters-12 Aldehydes, Ketones and Carboxylic Acids
| Section Name | Topic Name |
| 12 | Aldehydes, Ketones and Carboxylic Acids |
| 12.1 | Nomenclature and Structure of Carbonyl Group |
| 12.2 | Preparation of Aldehydes and Ketones |
| 12.3 | Physical Properties |
| 12.4 | Chemical Reactions |
| 12.5 | Uses of Aldehydes and Ketones |
| 12.6 | Nomenclature and Structure of Carboxyl Group |
| 12.7 | Methods of Preparation of Carboxylic Acids |
| 12.8 | Physical Properties |
| 12.9 | Chemical Reactions |
| 12.10 | Uses of Carboxylic Acids |
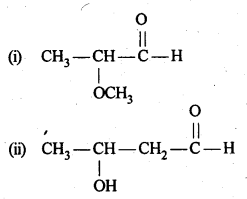
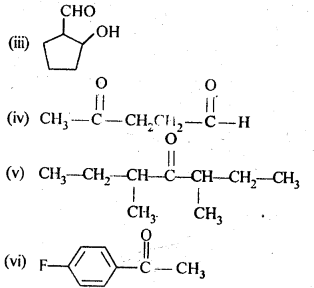
12.1. Write the structures of the following compounds:
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-OxopentanaI
(v) Di-sec.butylketone
(vi) 4-fluoroaeetophenone
Ans:

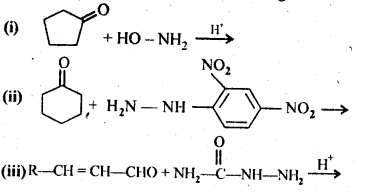
12.2. Write the structures of the products of the following reactions:
Ans:
12.3. Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Ans: The order is : CH3CH2CH3 < CH3OCH3 < CH3CHO <CH3CH2OH
All these compounds have comparable molecular masses CH3CH2OH undergoes extensive intermolecular Il-bonding and thus its b.pt. is the highest. CH3CHO is more pdlar than CH3OCH3 so that dipole-dipoie interactions in CH3CHO are greater than in CH3OCH3. Thus, b.pt. of CH3CHO > CH3OCH3. CH3CH2CH3 has only weak van der waals forces between its molecules and hence has the lowest b.pt.
12.4. Arrange the following carbonyl compounds in increasing order of their reactivity in nucleophilic addition reactions :
(a) Ethanal, propanal, propanone, butanone
(b) Benzaldehyde, p-tolualdehyde, p-nitrobenzaldehyde, acetophenone
Ans: (a) The increasing order of reactivity of the carbonyl compounds towards nucleophilic addition reactions is :
butanone < propanone < propanal < ethanal
The reactivity is based upon two factors. These are: steric factors and electronic factors.
(b) The increasing order of reactivity is :
acetophenone < p-tolualdehyde < benzaldehyde < p-nitrobenzaldehyde
Explanation: Acetophenone being a ketone is the least reactive towards nucleophilic addition. All others are aldehydes. Among them, p-tolualdehyde is less reactive than benzaldehyde because CH3 group present at the para position w.r.t. -CHO group will increase the electron density on the carbonyl carbon atom due to hyper conjugation effect. As a result, the nucleophile attack occurs to lesser extent as compared to benzaldehyde.
In p-nitrobenzaldehyde, the nitro group has an opposing effect. It is electron withdrawing in nature due to -I effect as well as -R effect. The electron density on the carbonyl carbon atom decreases and this favours the nucleophile attack.
12.5. Predict the products of the following reactions:
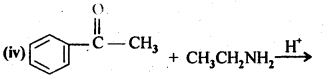
Ans:
12.6. Give the 1UPAC names of the following compounds:
(i) PhCH2CH2COOH
(ii) (CH3)2 C=CHCOOH
Ans: (i) 3 – Phenylpropanoic acid
(ii) 3 – Methylbut-2-enoic acid
(iii) 2-Methylcyclohexanecarboxylic acid
(iv) 2,4,6 – Trinitrobenzoic acid
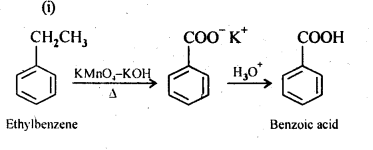
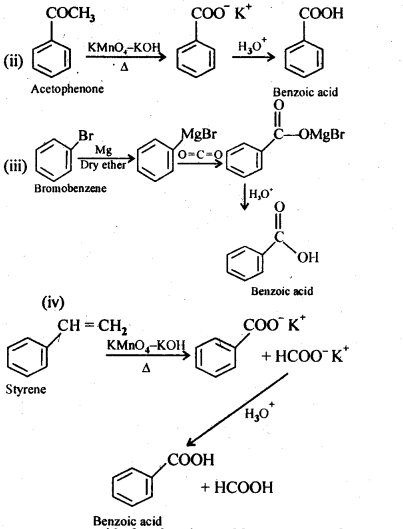
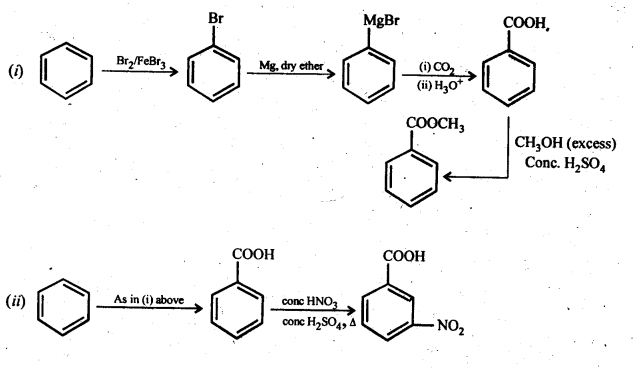
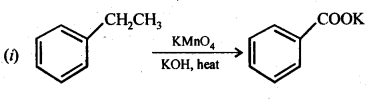
12.7. Show how each of the following compounds can be converted into benzoic acid.
(i) Ethylbenzene
(ii) Acetophenone
(iii) Bromobenzene
(iv) Phenylethene (styrene)
Ans:

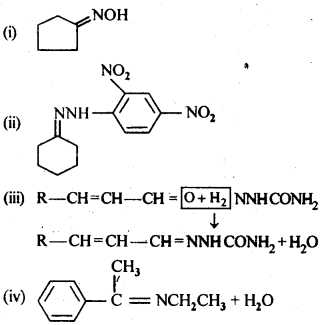
12.8. Which acid from each of the following pairs would you expect to be a stronger acid?
(i) CH3COOH or CH2FCOOH
(ii) CH2FCOOH or CH2ClCOOH
(iii) CH2FCH2CH2COOH or CH3CHFCH2COOH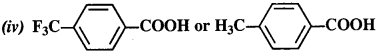
Ans:
Explanation: CH3 group with +I effect increases the electron density on the oxygen atom in O – H bond in the carboxyl group and cleavage of bond becomes diffcult. It therefore, decreases the acidic strength. The F atom has very strong -I effect, i.e., electron withdrawing influence. It decreases the electron density on the oxygen atom and cleavage of bond becomes easy. The acidic character therefore, increases. It is further related to the
- No. of F atoms present in the molecule.
- Relative position of the F atom in the carbon atom chain.
In the light of the above discussion.
(i) CH2FCOOH is a stronger acid.
(ii) CH2FCOOH is a stronger acid.
(iii) CH3CHFCH2COOH is a stronger acid.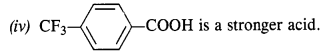
f
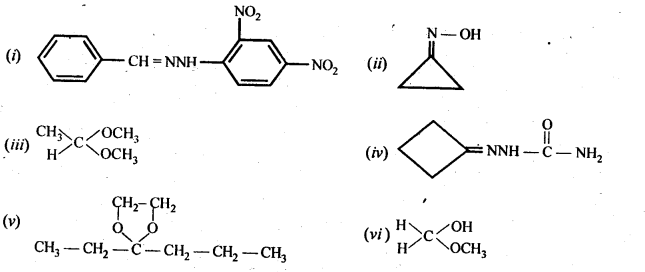
12.1. What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin
(ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(viii) Imine
(ix) 2,4-DNP derivative
(x) Schiff’s base.
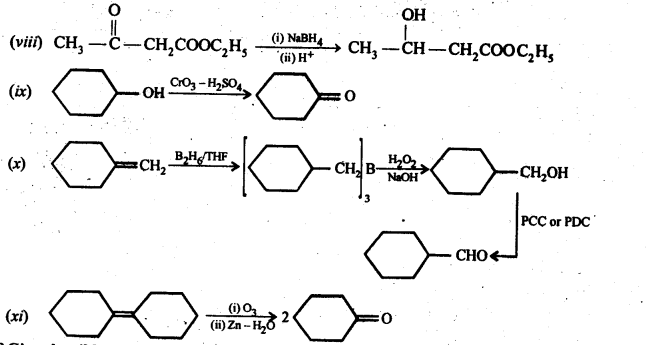
Ans: (i) Cyanohydrin: gem-Hydroxynitriles, i.e., compounds possessing hydroxyl and cyano groups on the same carbon atom are called cyanohydrins. These are produced by addition of HCN to aldehydes or ketones in a weakly basic medium.![]()
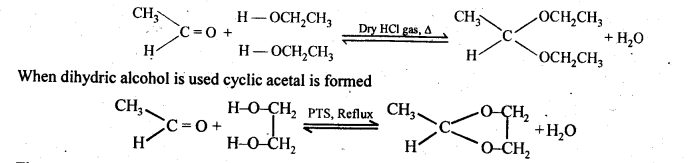
(ii) gem – Dialkoxy compounds in which the two alkoxy groups are present on the terminal carbon atom are called acetals. These are produced by the action of an aldehyde with two equivalents of a monohydric alcohol in presence of dry HCl gas.
These are easily hydrolysed by dilute mineral acids to regenerate the original aldehydes. Therefore, these are used for the protection of aldehyde group in organic synthesis.
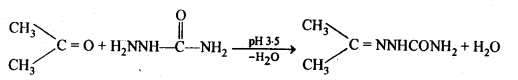
(iii) Semicarbazones are derivatives of aldehydes and ketones and are produced by action of semicarbazide on them in acidic medium.
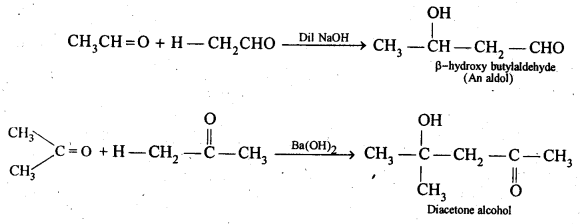
(iv) Aldols are P-hydroxy aldehydes or ketones and are produced by the condensation of two molecules of the same or one molecule each of two different aldehydes or ketones in presence of a dilute aqueous base. For example,
(v) gem – Alkoxyalcohols are called hemiacetals. These are produced by addition of one molecule of a monohydric alcohol to an aldehyde in presence of dry HCl gas.
(vi) Oximes are produced when aldehydes or ketones react with hydroxyl amine in weakly acidic medium.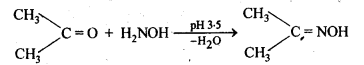
(vii) Ketals are produced when a ketone is heated with dihydric alcohols like ethylene glycol in presence of dry HCl gas or /3-toluene sulphonic acid (PTS).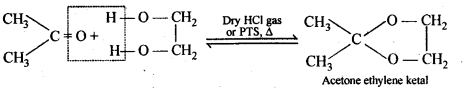
These are easily hydrolysed by dilute mineral acids to regenerate the original ketones. Therefore, ketals are used for protecting keto groups in organic synthesis.
(viii) Compounds containing -C = N – group are called imines. These are produced when aldehydes and ketones react with ammonia derivatives.![]()
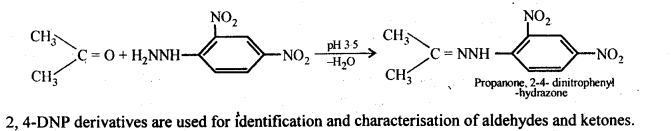
(ix)2, 4-Dinitrophenyl hydrazone (i.e., 2,4-DNP derivatives) are produced when aldehydes or ketones react with 2,4-dinitrophenyl hydrazine in weakly acidic medium.
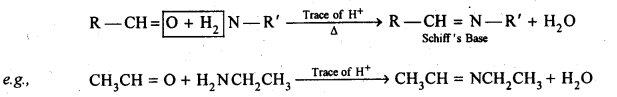
(x) Aldehydes and ketones react with primary aliphatic or aromatic amines to form azomethines or SchifFs bases.
12.2. Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH (CH3)—CH2 CH2—CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH.
(vii) OHCC6H4CHO-p
Ans: (i) 4-Methyl pentanal
(ii) 6-Chloro-4-ethylhexan-3-one
(iii) But-2-en-l-al
(iv) Pentane-2,4-dione
(v) 3,3,5-Trimethyl-hexan-2-one
(vi) 3,3-Dimethyl butanoic acid
(vii) Benzene-1,4-dicarbaldehyde
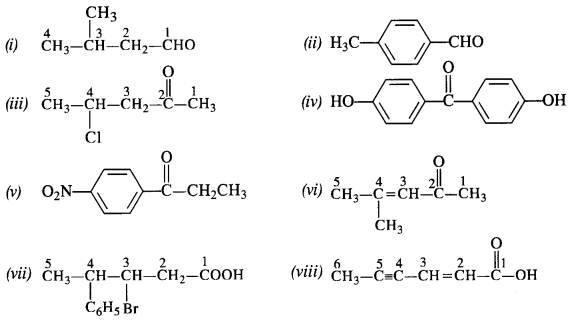
12.3. Draw the structures of the following compounds.
(i) 3-Methylbutanal
(ii) p-Methylbenzaldehyde
(iii) 4-Chloropentan-2-one
(iv) p, p’-Dihydroxybenzophenone
(v) p-Nitropropiophenone
(vi) 4-Methylpent-3-en-2-one.
(vii) 3-Bromo-4-phenylpentanoic acid
(viii) Hex-2-en-4-ynoic acid
Ans:
12.4. Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CH BrCH2CH(CH3)CHO
(iii) CH3(CH2)5CHO
(iv) Ph—CH=CH—CHO
Ans:
12.5. Draw structures of the following derivatives:
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cydopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Ans:
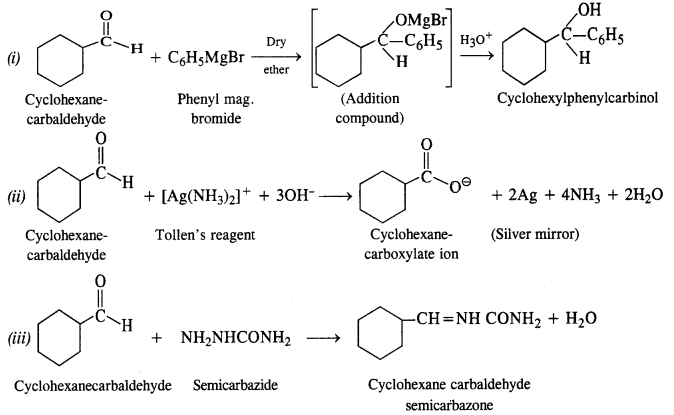
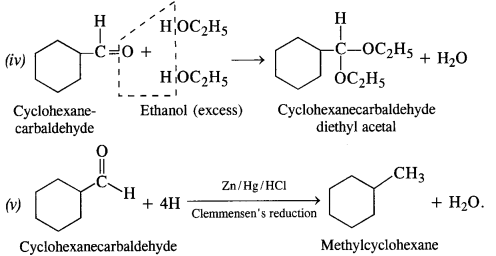
12.6. Predict the product when cyclohexanecarbaldehyde reacts with following reagents :
(i) C6H5MgBr followed by H30+
(ii) Tollen’s reagent
(iii) Semicarbazide in the weakly acidic medium
(iv) Excess of ethanol in the presence of acid
(v) Zinc amalgam and Cyclohexanecarbaldehyde Semicarbazide
Ans:

12.7. Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde.
(iv) Benzophenone
(v) Cyclohexanone
(vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde
(viii) Butan-l-ol 1
(ix) 2,2-Dimethylbutanal
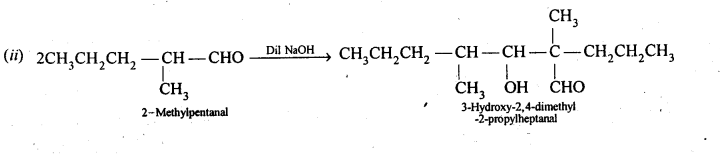
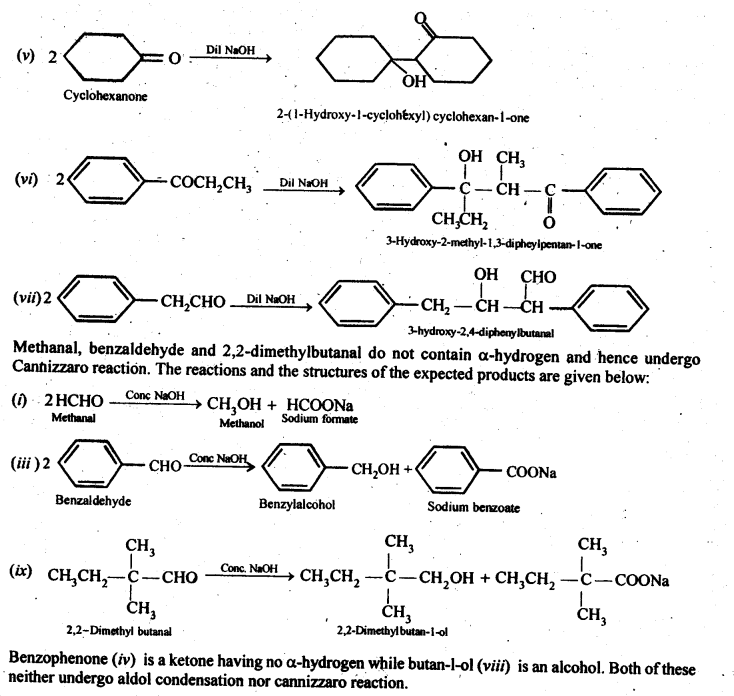
Ans: 2-Methylpertfanal, cyclohexanone, 1-phenylpropanone and phenylacetaldehyde contain one or more a-hydrogen and hence undergo aldol condensation. The reactions and the structures of the expected products are given below:

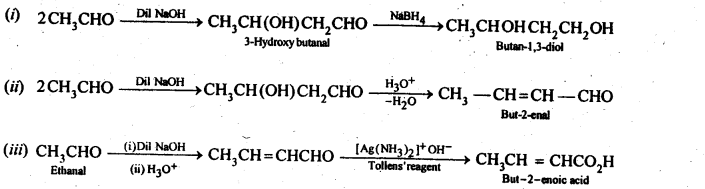
12.8. How will you convert ethanal into the following compounds?
(i) Butane-1,3-diol
(ii) But-2-enal
(iii) But-2-enoic acid
Ans:
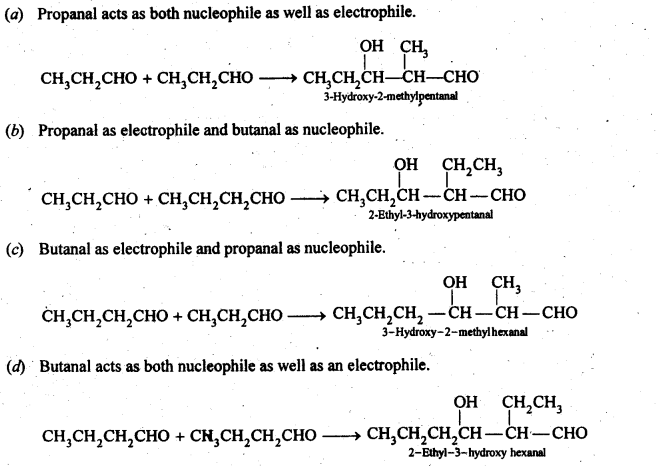
12.9. Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Ans:
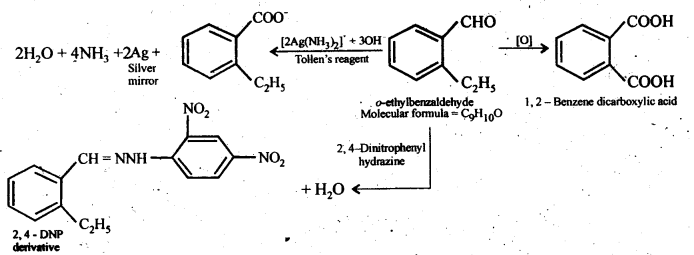
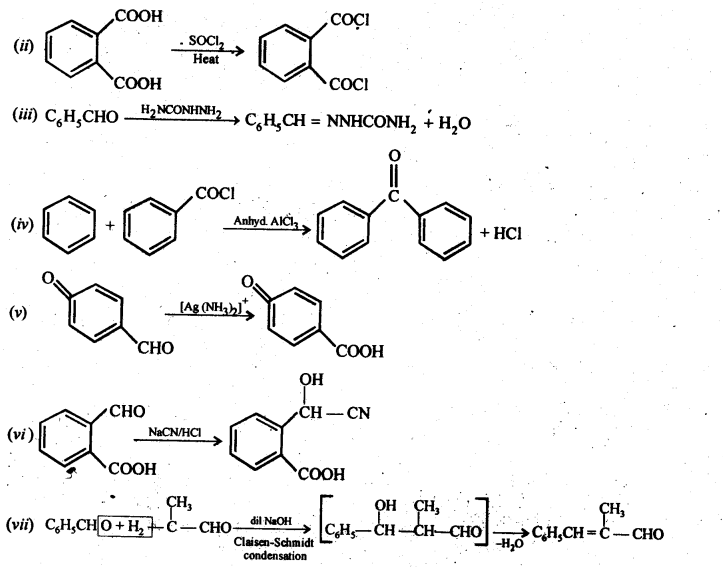
12.10. An organic compound with the molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollen’s reagent, and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
Ans: Since the given compound with molecular formula C9H10O forms a 2,4-DNP derivative and reduces Tollen’s reagent, it must be an aldehyde. Since it undergoes Cannizzaro reaction, therefore, CHO group is directly attached to die benzene ring.
Since on vigorous oxidation, it gives 1, 2-benzene dicarboxylic acid, therefore, it must be an ortho- substituted benzaldehyde. The only o-substituted aromatic aldehyde having molecular formula C9H10O is o-ethyl benzaldehyde. Ail the reactions can now be explained on the basis of this structure.
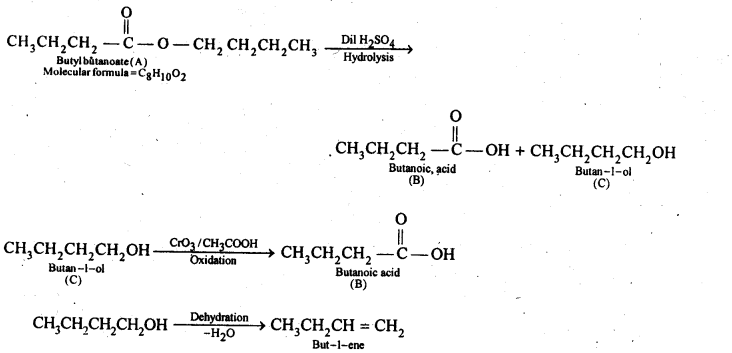
12.11. An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B} and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (Q on dehydration gives but-l-ene. Write equations for the reactions involved.
Ans: Since an ester A with molecular formula C8H16O2 upon hydrolysis gives carboxylic acid B and the alcohol C and oxidation of C with chromic acid produces the acid B, therefore, both the carboxylic acid B and alcohol C must contain the same number of carbon atoms.
Further, since ester A contains eight carbon atoms, therefore, both the carboxylic acid B and the alcohol C must contain four carbon atoms each.
Since the alcohol C on dehydration gives but-l-ene, therefore, C must be a straight chain alcohol, i.e., butan-l-ol.
If C is butan-l-ol, then the acid B must be butanoic acid and the ester A must be butyl butanoate.The chemical equations are as follows:
12.12. Arrange the following in increasing order of the property indicated :
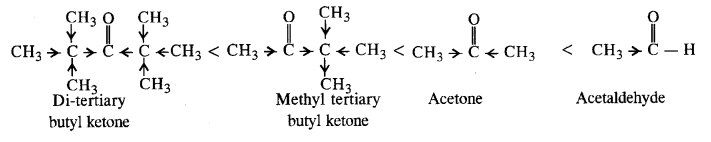
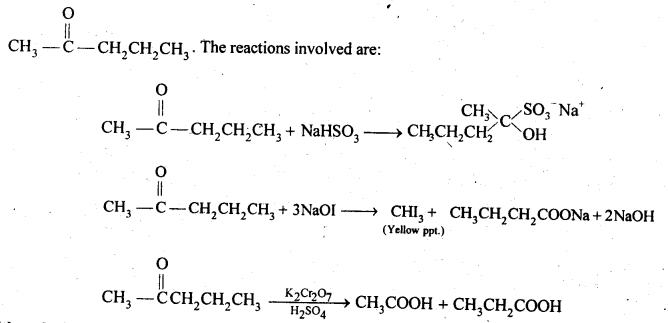
(i) Acetaldehyde, Acetone, Di tert. butyl ketone, Methyl tert. butyl ketone (reactivity towards HCN). (C.B.S.E. Sample Paper 2011, 2015, C.B.S.E. Delhi 2012)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength) (C.B.S.E. Delhi2008)
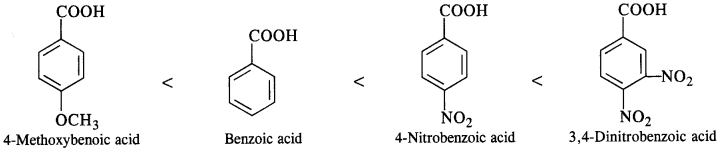
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3, 5-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength) (C.B.S.E. Sample Paper 2011, 2015; C.B.S.E. Delhi 2012, C.B.S.E. Outside Delhi 2015, Rajasthan Board 2015)
Ans: (i) Cyanohydrin derivatives are formed as a result of the reaction in which the nucleophile (CN– ion) attacks the carbon atom of the carbonyl group. The order of reactivity
- decreases with increase in +I effect of the alkyl group.
- decreases with increase in steric hindrance due to the size as well as number of the alkyl groups. In the light of the above information, the decreasing order of reactivity is :

(ii) We know that alkyl group with +I effect decreases the acidic strength. The +I effect of isopropyl group is more than that of n-propyl group. Similarly, bromine (Br) with -I-effect increases the acidic strength. Closer its position in the carbon atom chain w.r.t., carboxyl (COOH) group, more will be its -I-effect and stronger will be the acid. In the light of this, the increasing order of acidic strength is :
(CH3)2CHCOOH< CH3CH2CH2COOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br) COOH
(iii) We have learnt that the electron donating group (OCH3) decreases the acidic strength of the benzoic acid. At the same time, the electron withdrawing group (N02) increases the same. Keeping this in mind, the increasing order of acidic strength is:
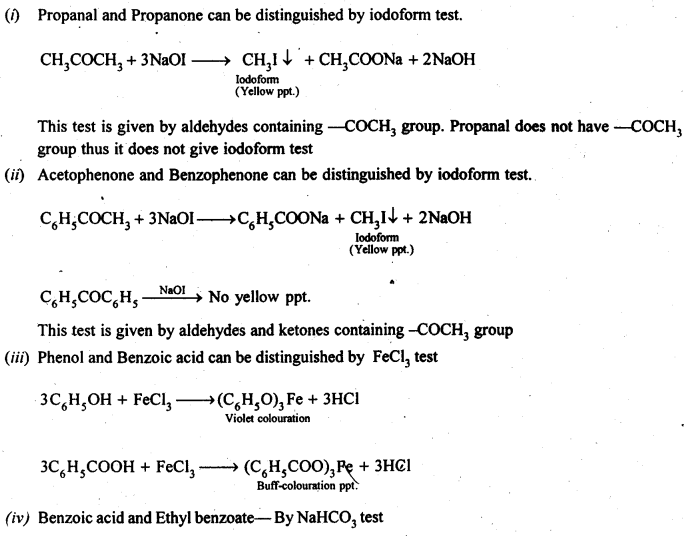
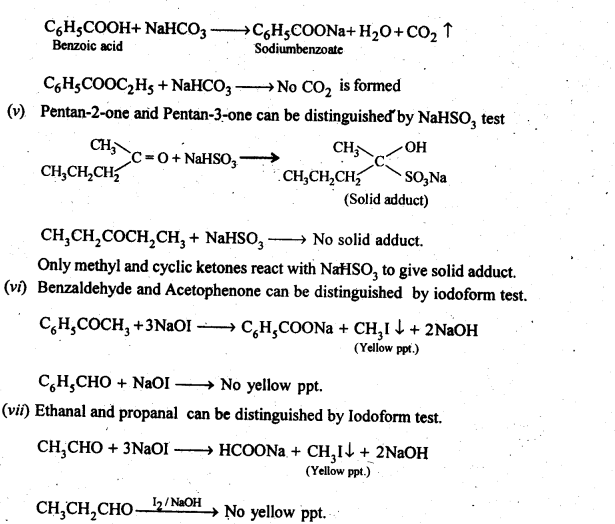
12.13. Give simple chemical tests to distinguish between the following pairs of compounds.
(i) PropanalandPropanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone.
(vii) EthanalandPropanal
Ans:

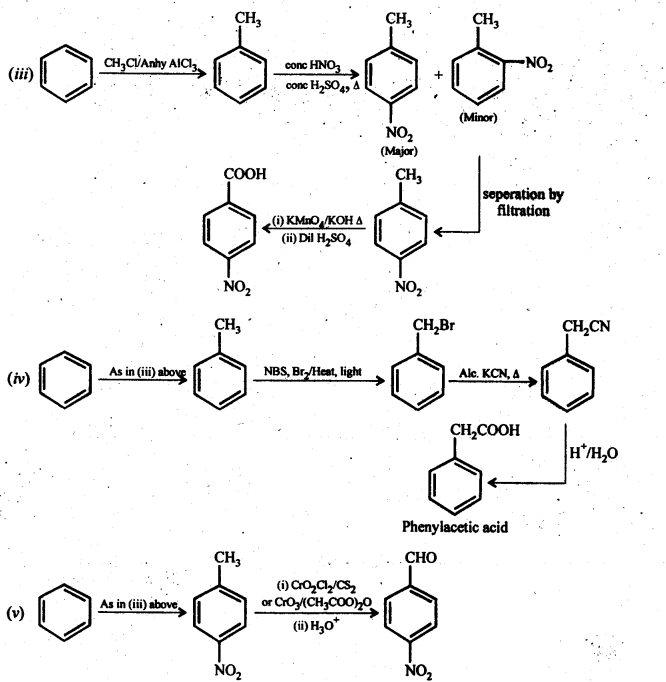
12.14. Row will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
(i) Methyl benzoate
(ii) m-nitrobenzoic acid
(iii) p-nitrobenzoic acid
(iv) Phenylaceticacid
(v) p-nitrobenzaldehyde
Ans:

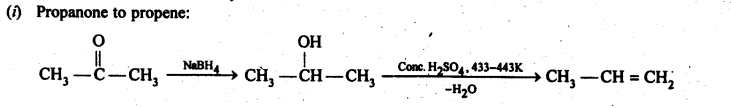
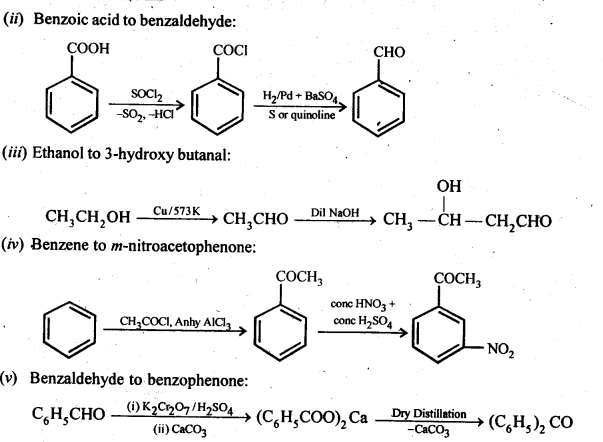
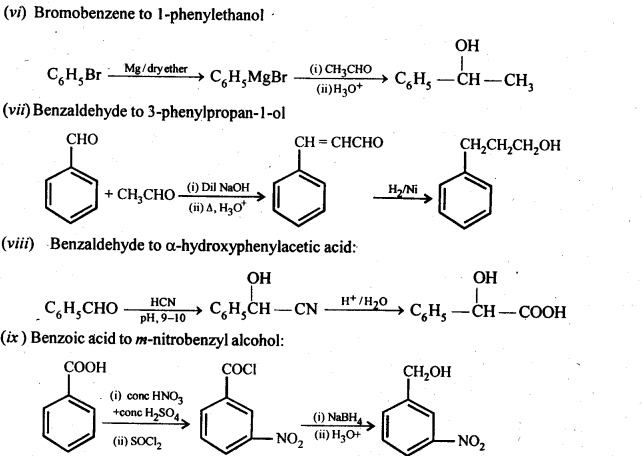
12.15. How will you bring about the following conversions in not more than two steps?
(i) PropanonetoPropene
(ii) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(iv) Benzene to m-Nitroacetophenone
(v) Benzaldehyde to Benzophenone –
(vi) Bromobenzeneto 1-PhenylethanoL
(vii) Benzaldehyde to 3-Phenylpropan-1-ol.
(viii) Benzaldehyde to α Hydroxyphenylacetk acid
(ix) Benzoic acid to m-Nitrobenzy 1 alcohol
Ans:


12.16. Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
Ans: (i) Acetylation refers to the process of introducing an acetyl group into a compound namely, the substitution of an acetyl group for an active hydrogen atom. Acetylation is usually carried out in presence of a base such as pyridine, dimethylanitine, etc.![]()
(ii) Cannizzaro reaction : Aldehydes which do not contain an a-hydrogen atom, when treated with concentrated alkali solution undergo disproportionation, i.e., self oxidation reduction. As a result, one molecule of the aldehyde is reduced to the corresponding alcohol at the cost of the other which is oxidised to the corresponding carboxylic acid. This reaction is called Cannizzaro reaction.
(iii) Cross aldol condensation: Aldol condensation between two different aldehydes is called cross aldol condensation.If both aldehydes contain a-hydrogens, It gives a mixture of four products.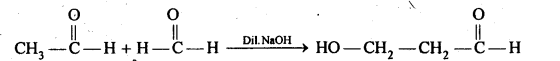
(iv) Decarboxylation: The process of removal of a molecule of CO2 from a carboxylic acid is called decarboxylation. Sodium salts of carboxylic acids when heated with soda-lime undergoes decarboxylation to yield alkanes.![]()
12.17. Complete each synthesis by giving missing starting material, reagent or products.
Ans:





12.18. Give plausible explanation for each of the following:
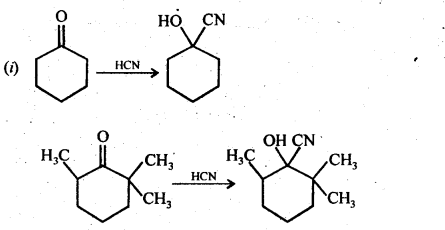
(i) Cyclohexanone forms cyanohydrin in good yield but 2,2, fctrimethylcyclohexanone does not
(ii) There are two – NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii)During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Ans:
The yield of second reaction is very low because of the presence of three methyl groups at ex-positions with respect to the C = O, the nucleophilic attack by the CN– ion does not occur due to steric hinderance. Since there is no such steric hindrance in cyclohexanone, therefore, nucleophilic attack by the CN– ion occurs readily and hence cyclohexanone cyanohydrin is obtained in good yield.
Although semicarbazide has two – NH2 groups but one of them (i.e., which is directly attached to C = O) is involved in resonance as shown above. As a result, electron density on N of this -NH2 group decreases and hence it does not act as a nucleophile. In contrast, the other -NH2 group (i.e.. attached to NH) is not involved in resonance and hence lone pair of electrons present on N atom of this -NH2 group is available for nucleophilic attack on the C = O group of aldehydes and ketones.’
(iii) The formation of esters from a carboxylic acid and an alcohol in presence of an acid catalyst is a reversible reaction.![]()
Thus to shift the equilibrium in the forward direction, the water or the ester formed should be removed as fast as it is formed.
12.19. An organic compound contains 69-77% carbon, 11-63 % hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tottens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vigorous oxidation, it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Ans:
Since the compound form sodium hydrogen sulphite addition product, therefore, it must be either an – aldehyde or methyl/ cyclic ketone. Since the compound does not reduce Tollens’ reagent therefore, it cannot be an aldehyde. Since the compound gives positive iodoform test, therefore, the given compound is a methyl ketone. Since the given compound on vigorous oxidation gives a mixture ofethanoic acid and propanoic acid, therefore, the methyl ketone is pentan-2-one, i.e.,
12.20. Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than on phenol. Why?
Ans: Consider the resonating structures of carboxylate ion and phenoxide ion.
In case of phenoxide ion, structures (V – VII) carry a negative charge on the less electronegative carbon atom.Therefore, their contribution towards the resonance stabilization of phenoxide ion is very small.
In structures I and II, (carboxylate ion), the negative charge is delocalized over two oxygen atoms while in structures III and IV, the negative charge on the oxygen atom remains localized only the electrons of the benzene ring are delocalized. Since delocalization of benzene electrons contributes little towards the stability of phenoxide ion therefore, carboxylate ion is much more resonance stabilized than phenoxide ion. Thus, the release of a proton from carboxylic acids is much easier than from phenols. In other words, carboxylic acids are stronger acids than phenols.
Coordination Compounds
| Section Name | Topic Name |
| 9 | Coordination Compounds |
| 9.1 | Werner’s Theory of Coordination Compounds |
| 9.2 | Definitions of Some Important Terms Pertaining to Coordination Compounds |
| 9.3 | Nomenclature of Coordination Compounds |
| 9.4 | Isomerism in Coordination Compounds |
| 9.5 | Bonding in Coordination Compounds |
| 9.6 | Bonding in Metal Carbonyls |
| 9.7 | Stability of Coordination Compounds |
| 9.8 | Importance and Applications of Coordination Compounds |
9.1. Write the formulas for the following coordination compounds:
(i)Tetraamminediaquacobalt(IlI) chloride
(ii)Potassium tetracyanidonickelate(II)
(iii)Tris(ethanp-1,2-diamine) chromium(III) chloride
(iv)Amminebromidochloridonitrito-N- platinatc(II)
(v)Dichloridobis(ethane-l ,2-diamine) platinum (IV) nitrate
(vi)Iron(III)hexacyanidoferrate(II)
Ans: (i) [CO(NH3)4(H2O)2]Cl3.
(ii)K2[Ni(CN)4]
(iii)[Cr(en)3]Cl3
(iv)[Pt (NH3) Br Cl (N02)]–
(v)[PtCl2(en)2](N03)2
(vi)Fe4[Fe(CN)6]3
9.2. Write IUPAC names of following co-ordination compounds :
(a) [CO(NH3)6]Cl3
(b) [CO(NH3)Cl]Cl2
(C) K3[Fe(CN)6]
(d) [K3[Fe(C2O4)3]
(e) K2[PdCl4]
(f) [Pt(NH3)2ClNH2CH3]Cl. (C. B. S. E. Delhi2013)
Ans:
(a) hexaamminecobalt (III) chloride
(b) pentaamminechloridocobalt (III) chloride
(c) potassium hexacyanoferrate (III)
(d) potassium trioxalatoferrate (III)
(e) potassium tetrachloridoplatinum (II)
(f) diamminechlorido (methylamine) platinum(II) chloride.
9.3. Indicate the types of isomerism exhibited by the . . following complexes and draw the structures for these isomers:
(i)K[Cr(H2O)2(C2O4)2]
(ii)[CO(en)3]Cl3
(iii)[CO(NH3)5(NO2)(NO3)2], .
(iv)[Pt(NH3)(H2O)Cl2]
Ans: (i)(a) geometrical isomerism (cis and tram)
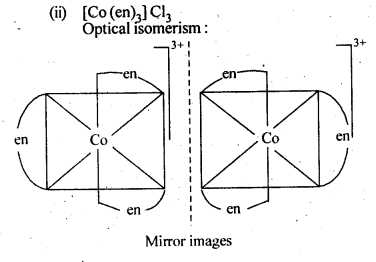
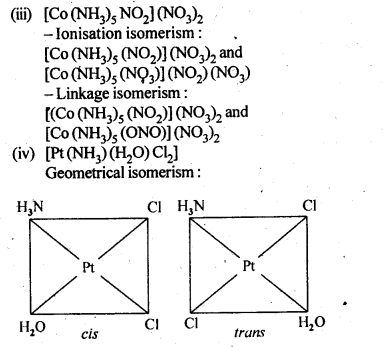
9.4. Give evidence that [Co(NH3)5Cl]S04 and [Co(NH3)5S04]Cl are ionisation isomers.
Ans: When dissolved in water, they give different ions in solution which can be tested by adding AgN03 solution and BaCl2 solution, i.e.,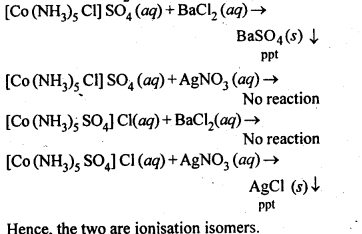
9.5. Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square planar structure is diamagnetic and [NiCl4]2- ion with tetrahedral geometry is paramagnetic. (Rajasthan Board 2012)
Ans: Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2. Nickel in this complex is in + 2 oxidation state. It achieves + 2 oxidation state by the loss of the two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CN– ion is a strong field, under its attacking influence, two unpaired electrons in the 3d orbitals pair up.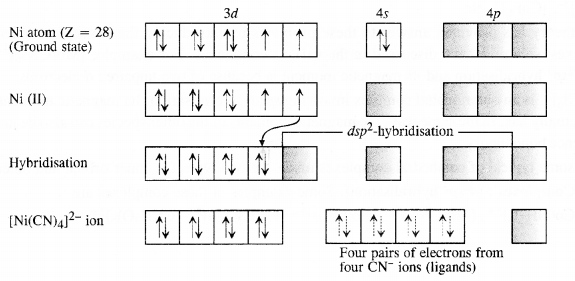
Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2 Nickel in this complex is in + 2 oxidation state. Nickel achieves + 2 oxidation state by the loss of two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CP ion is a weak field ligand, it is not in a position to cause electron pairing.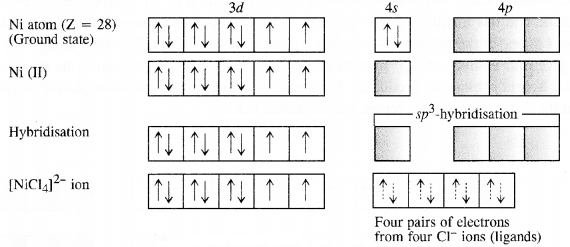
9.6. [NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedraL Why?
Ans: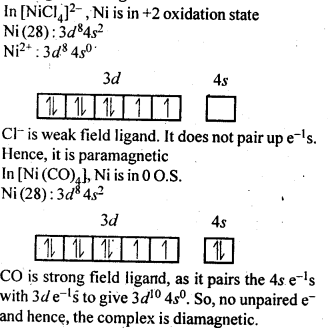
9.7. [Fe(H2O)6]3+is strongly paramagnetic whereas [Fe(CN)6]3-is weakly paramagnetic. Explain.
Ans: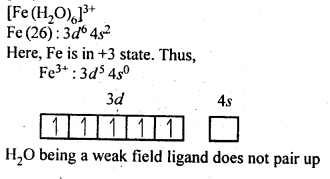
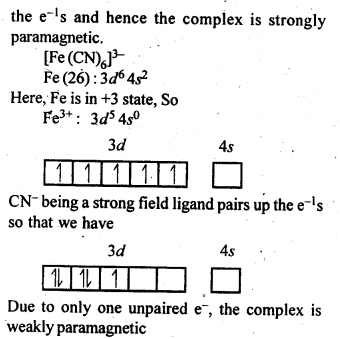
9.8. Explain[CO(NH3)6]2+ is an inner orbital complex.whereas [Ni(NH3)6]2+ is an outer orbital complex.
Ans: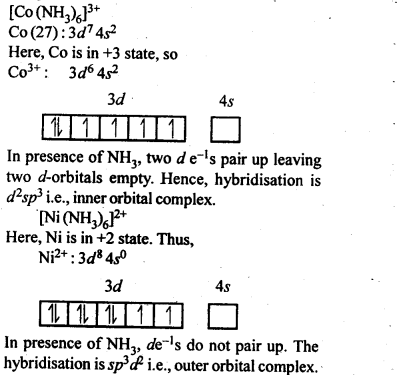
9.9. Predict the number of unpaired electrons in the square planar [Pt(CN)4]2- ion.
Ans: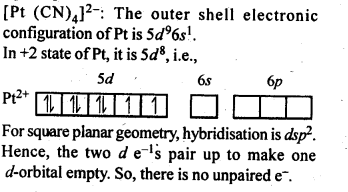
9.10. The hexaaquamanganese (II) ion contains five unpaired electrons while the hexacyano ion contains only one unpaired electron. Explain using crystal field theory.
Ans: Mn(II) ion has 3d5 configuration. In the presence of H2O molecules acting as weak field ligands, the distribution of these five electrons is
9.11. Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ ion, given that β4 for this complex is 2.1 x 1013.
Ans: Overall stability constant (β4) = 2.1 x 1013.
Thus, the overall dissociation constant is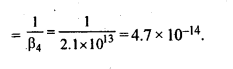
9.1. Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans: The main postulates of Werner’s theory of coordination compounds are as follows:
(a)Metals possess two types of valencies called
(i) primary valency which are ionisable; (ii) secondary valency which are non- ionisable
(b)Primary valency is satisfied by the negative ions and it is that which a metal exhibits in the formation of its simple salts.
(c)Secondary valencies are satisfied by neutral ligand or negative ligand and are those which metal exercises in the formation of its complex ions. Every cation has a fixed number of secondary valencies which are directed in space about central metal ion in certain fixed directions, e.g„ In CoCl3-6NH3, valencies between Co and Cl are primary valencies and valencies between Co and NH3 are secondary. In COCl3-6NH3 , six ammonia molecules linked to Co by secondary valencies are directed to six corners of a regular octahedron and thus account for structure of COCl3-6NH3 as follows:
In modern theory, it is now referred as coordination number of central metal atom or ion.
9.2. FeSO4 solution mixed with (NH4)2SO4 solution in 1 : 1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1 : 4 molar ratio does not give the test of Cu2+ ion. Explain why?
Ans: When FeSO4 and (NH4)2SO4 solutions are mixed in 1 : 1 molar ratio, a double salt known as Mohr’s salt is formed. It has the formula FeSO4.(NH4)2SO4.6H2O. In aqueous solution, the salt dissociates as :![]()
The solution gives the tests for all the ions including Fe2+ ions. On the other hand, when CuSO4 and NH3 are mixed in the molar ratio of 1 : 4 in solution, a complex [Cu(NH3)4]SO4 is formed. Since the Cu2+ ions are a part of the complex entity (enclosed in square bracket), it will not give their characteristic tests as are given by Fe2+ ions.
9.3. Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: Coordination entity: It constitutes of a central atom/ion bonded to fixed number of ions or molecules by coordinate bonds e.g. [COCl3(NH3)3], [Ni (CO)4] etc.
Ligand : The ions/molecules bound to central atom/ion in coordination entity are called ligands. Ligands in above examples are CL, NH3, CO Coordination number : This is the number of bond formed by central atom/ion with ligands. Coordination polyhedron : Spatial arrangement of ligands defining the shape of complex. In above cases Co and Ni polyhedron are octahedral and tetrahedral in [CoCl3 (NH3)3] and [Ni(CO)4] respectively.
Homoleptic : Metal is bound to only one kind of ligands eg Ni in[Ni(CO)4]
Heteroletric Metal is bound to more than one kind of ligandseg Coin [CoCl3(NH3)3]
9.4. What is meant by unidentate didentate and ambidentate ligands? Give two examples for each.
Ans: A molecule or an ion which has only one donor atom to form one coordinate bond with the central metal atom is called unidentate ligand, e.g,, Cl- and NH3.
A molecule or ion which contains two donor atoms and hence forms two coordinate bonds with the central metal atom is called adidentate
A molecule or an ion which contains two donor atoms but only one of them forms a coordinate bond at a time with the central metal atom is called![]()
9.5. Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+ (ii) [CoBr2(en)2]+ (iii) [PtCl4]2- (iv) K3[Fe(CN)6] (v) [Cr(NH3)3CI3]
Ans: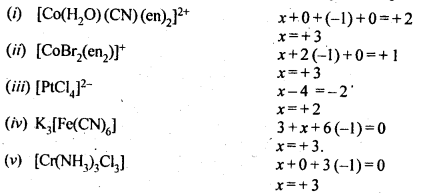
9.6. Using IUPAC norms, write the formulae for the following : (C.B.S.E. Foreign 2015)
(a) tetrahydroxozincate(II)
(b) hexaammineplatinum (TV)
(c) potassiumtetrachloridopalladate(II)
(d) tetrabromidocuprate (II)
(e) hexaaminecobalt(III) sulphate
(f) potassiumtetracyanonicklate (II)
(g) potassiumtrioxalatochromate(III)
(h) pentaamminenitrito-O-cobalt(III)
(i) diamminedichloridoplatinum(II)
(j) pentaamminenitrito-N-cobalt (III). (C.B.S.E. Delhi 2012)
Ans: (a) [Zn(OH)4]2-
(b) [Pt(NH3)6]4+
(c) K2[PdCl4]
(d) [Cu(Br)4]2-
(e) [CO(NH3)6]2 (SO4)3
(f) K2[Ni(CN)4]
(g) K3 [Cr(OX)3]
(h) [CO(NH3)5ONO]2+
(i) [Pt(NH3)2Cl2]
(j) [CO(NH3)5NO2]2+.
9.7. Using IUPAC norms write the systematic names of the following:
(i) [Co(NH3)6]CI3,
(ii)[Pt(NH3)2CI (NH2CH3)] Cl
(iii) [Ti(H20)6]3+
(iv) [Co(NH3)4Cl(N02)]CI
(v)|Mn(H20)6]2+
(vi)[NiCl4]2-
(vii)[Ni(NH3)6]CI2
(viii)[Co(en)3]3+
(ix) [Ni(CO)4]
Ans: (i) Hexaammine cobalt (III) chloride.
(ii) Diammine chlorido (methylamine) platinum (II) chloride.
(iii) Hexaaquatitanium (III) ion.
(iv) Tetraammine chlorido nitrito-N-cobalt (IV) chloride.
(v)Hexaaquamanganese (II) ion.
(vi)Tetrachloridonickelate (II) ion.
(vii)Hexaammine nickel (II) chloride.
(viii)Tris (ethane -1,2-diamine) cobalt (III) ion.
(ix) Tetra carbonyl nickel (0).
9.8. List various types of isomerism possible for coordination compounds, giving an example of each.
Ans: Coordination compounds exhibit stereo isomerism and structural isomerism.
Two types of stereoisomerism and their examples are as follows.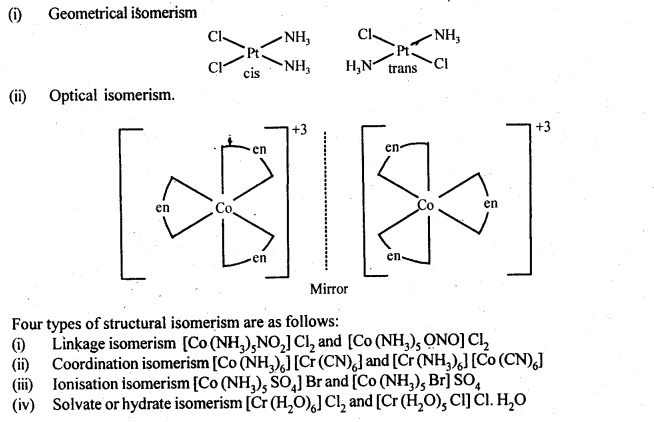
9.9. How many geometrical isomers are possible in . the following coordination entities?
(i) [Cr(C2O4)3]3- (ii) [CoCl3(NH3)3]
Ans: (i) [Cr(C2O4)3]3- => No geometrical isomers
are possible in this coordination entity.
(ii) [Co(NH3)3 Cl3] => Two geometrical isomers are possible (fac and mer) in this coordination entity.
9.10. Draw the structures of optical isomers of
(i) [Cr(C2O4)3]3-
(ii)[PtCI2(en)2]2+
(iii)[Cr(NH3)2CI2(en)]+
Ans: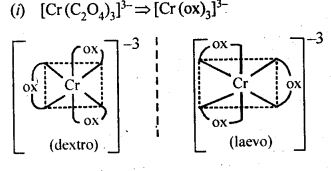
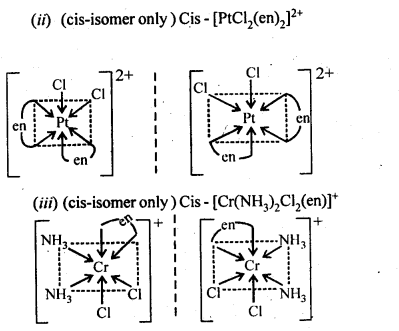
9.11. Draw all the isomers (geometrical and optical) of
(i)[CoCl2(en)2]+
(ii)[Co(NH3) Cl (en)2]2+
(iii) [Co(NH3)2Cl2(en)]+
Ans: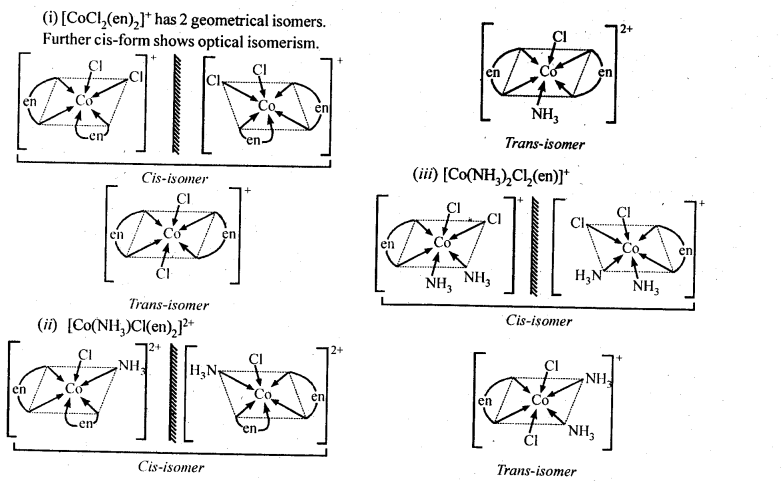
9.12. Write all the geometrical isomers of [Pt(NH3)(Br)(Cl) (Py)] and how many of these will exhibit optical isomerism?
Ans: Three isomers of[Pt(NH3)(Br)(Cl)(Py)] are possible.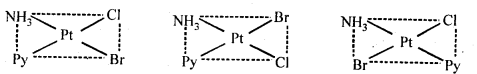
These are obtained by keeping the position of one of the ligand, say NH3 fixed and rotating the positions of others. This type of isomers do not show any optical isomerism. Optical isomerism only rarely occurs in square planar or tetrahedral complexes and that too when they contain unsymmetrical chelating ligand.
9.13. Aqueous copper sulphate solution (blue in colour) gives: (i) a green precipitate with aqueous potassium fluoride and (ii)a bright green solution with aqueous potassium chloride. Explain these experimental results.
Ans: Aqueous CuS04 solution exists as [Cu(H20)4]S04 which has blue colour due to [Cu(H20)4]2+ ions.
(i) When KF is added, the weak H20 ligands are replaced by F– ligands forming [CUF4]2- ions which is a green precipitate.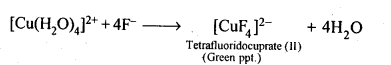
(ii)When KCl is added, Cl– ligands replace the weak H20 ligands forming [CuCl4]2- ion which has bright green colour.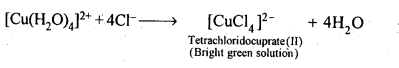
9.14. What is the coordination entity formed when excess of aqueons KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S (g) is passed through this solution?
Ans: First cupric cyanide is formed which decomposes to give cuprous cyanide and cyanogen gas. Cuprous cyanide dissolves in excess of potassium cyanide to form the complex, K3[Cu(CN)4],
Thus, coordination entity formed in the above reaction is [Cu(CN)4]3-. As CN– is a strong ligand, the complex ion is highly stable and does not dissociate/ionize to give Cu2+ ions. Hence, no precipitate,with H2S is formed.
9.15. Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3-
(iv) [CoF6]3-
Ans: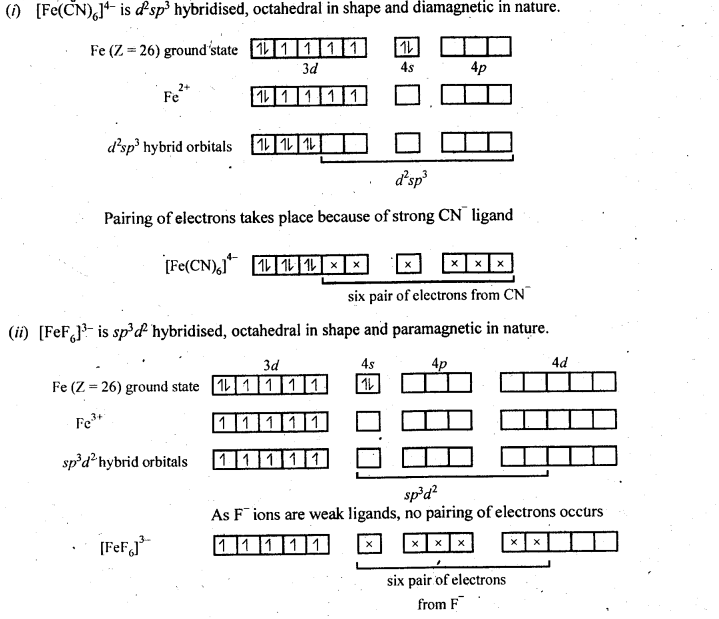
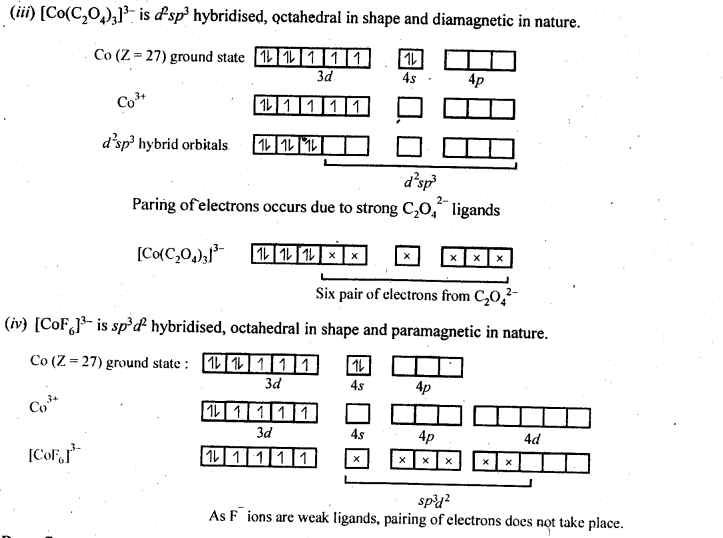
9.16. Draw figure to show the splitting of d-orbitals in an octahedral crystal field.
Ans: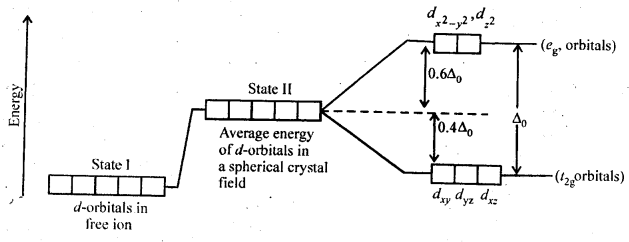
9.17. What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans: The crystal field splitting, ∆0, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d-orbitals. In general, ligands can be arranged in a series in the order of increasing field strength as given below :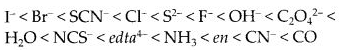
9.18. What is crystal field splitting energy? How does the magnitude of Δ0 decide the actual configuration of d-orbitals in a coordination entity?
Ans: When the ligands approach a transition metal ion, the d-orbitals split into two sets, one with lower energy and the other with higher energy. The difference of energy between the two sets of orbitals is called crystal field splitting energy (Δ0 for octahedral field). If Δ0 < P (pairing energy), the fourth electron enters
one of the e°g, orbitals giving the configuration t32ge1g, thus forming high spin complexes. Such ligands for which Δ0 < P are called weak field ligands. If Δ0 > P, the fourth electron pairs up in one of the t2g orbitals giving the configuration t42ge1g thereby forming low spin complexes. Such ligands for which Δ0> P are called strong field ligands.
9.19. [Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2- is diamagnetic. Explain why?
Ans: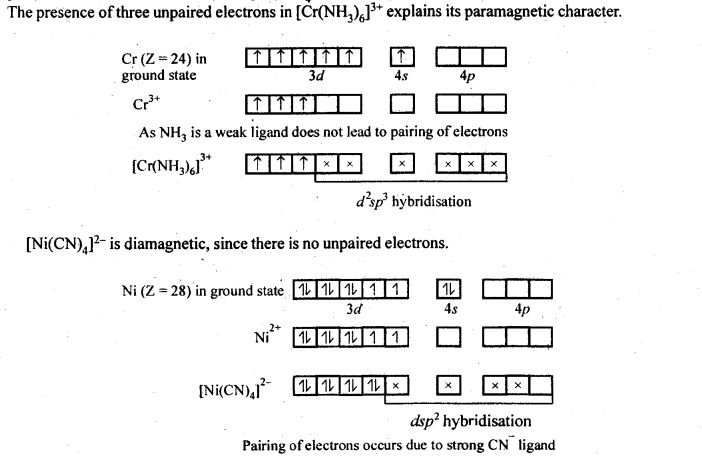
9.20. A solution of [Ni(H20)6]2+ is green but a solution of [Ni(CN)4]2-is colourless. Explain.
Ans: In [Ni(H20)6]2+, Ni is in + 2 oxidation state and having 3d8 electronic configuration, in which there are two unpaired electrons which do not pair in the presence of the weak H20 ligand. Hence, it is coloured. The d-d transition absorbs red light and the complementary light emitted is green.
In [Ni(CN)4]2- Ni is also in + 2 oxidation state and having 3d8 electronic configuration. But in presence of strong ligand CN– the two unpaired electrons in the 3d orbitals pair up. Thus, there is no unpaired electron present. Hence, it is colourless.
9.21. [Fe(CN)6]4- and [Fe(H2O)6]2+ are of different colours in dilute solutions. Why?
Ans: In both the complexes, Fe is in + 2 oxidation state with d6 configuration. This means that it has four unpaired electrons.Both CN– ion and H2O molecules which act as ligands occupy different relative positions in the spectrochemical series. They differ in crystal field splitting energy (∆0). Quite obviously, they absorb radiations corresponding to different wavelengths/frequencies from the visible region of light. (VIBGYOR) and the transmitted colours are also different. This means that the complexes have different colours in solutions.
9.22. Discuss the nature of bonding in metal carbonyls.
Ans: In metal carbonyl, the metal carbon bond (M – C) possess both the σ and π -bond character. The bond are formed by overlap of atomic orbital of metal with that of C-atom of carbon monoxide in following sequence:
(a)σ -bond is first formed between metal and carbon when a vacant d-orbital of metal atom overlaps with an orbital containing lone pair of electrons on C-atom of carbon monoxide (: C = O:)
(b)In addition to σ -bond in metal carbonyl, the electrons from filled d-orbitals of a transition metal atom/ ion are back donated into anti bonding π-orbitals of carbon monoxide. This stabilises the metal ligand bonding. The above two concepts are shown in following figure:
9.23. Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes:
(i)K3[CO(C2O4)3I (ii) cis-[Cr(en)2Cl2]Cl (iii) (NH4)2[CoF4] (iv) [Mn(H20)6]SO4
Ans: (i) K3[Co(C2O4)3] =>[CO(C204)3]3-. x + 3 (-2) = -3 . Oxidation state, x=+3 Coordination number is also 6 as C2042- is didentate. Co+3 is a case in which all electrons are paired
(ii) cis – [Cr(en)2Cl2]+ Cl–
x + 0—2 =+1
Oxidation state, x =+3
Coordination number is 6 as ‘en’ is didentate. Cr3+ is a cfi case, paramagnetic.
(iii) (NH4)2[COF4] = (NH4)22+[COF4]2-
x —4 =—2.
Oxidation state, x = + 2
Coordination number=4.
Co2+ is a d5 case, paramagnetic
(iv)[Mn(H20)6]2+S042-
x+0f+2
.•. Oxidation state, x- + 2
Coordination number is 6.
Mn+2 is a d5 case, paramagnetic
9.24. Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H20)2(C204)2|-3H20 (ii) [Co(NH3)5CIlCl2 (iii) CrCI3(Py)3
(iv)Cs[FeCl4] (v)K4|Mn(CN)6|
Ans: (i) K[Cr(H20)2(C204)2|-3H20 IUPAC name is potassiumdiaquadioxalatochromate (III) hydrate.
Coordination number = 6
Oxidation state of Cr: x + 0 + 2 (-2) = – 1
.‘. x = + 3
Shape is octahedral Electronic configuration of Cr3+ = 3d3=t32ge°g .
Magnetic moment,
= 3-87 BM
(ii) [Co(NH3)5CIlCl2IUPAC name is pentaamminechloridocobalt (III) chloride Coordination number of Co = 6 Shape is octahedral.
Oxidation state of Co, x + 0 -1 = + 2 .’. x = + 3
Electronic configuration of Co3+ = 3d6 = t62ge°g n=0, μ =0 .
(iii) CrCI3(Py)3. IUPAC name is
trichloridotripyridine chromium (III).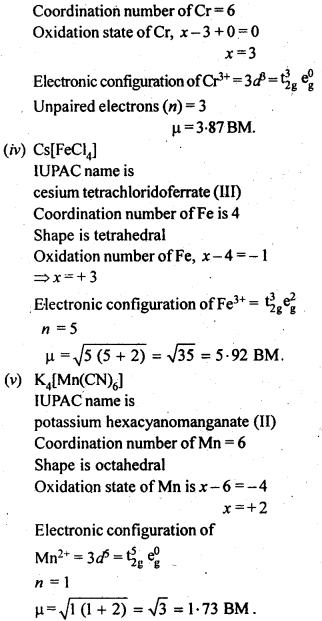
9.25. What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Ans: Formation of a complex in solution is an equilibrium reaction. It may be represented as
The equilibrium constant of this reaction is the measure of stability of the complex. Hence the equilibrium constant is also called as stability constant or Instability constant may be defined as equilibrium constant for reverse reaction. The formation of above complex may also be written in successive steps:
Stability constant is written as
β4=K1K2K3K4.
Greater the stability constant, stronger is the metal-ligand bond.
The stability of complex will depend on
(a)nature of metal
(b)Oxidation state of metal
(c)Nature of ligand e g. chelating ligand form stabler complexes
(d)Greater the basic strength of the ligand, more will be the stability.
9.26. What is meant by the chelate effect? Give an example.
Ans: When a didentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metal ion, a five or a six membered ring is formed, the effect is called chelate effect. For example,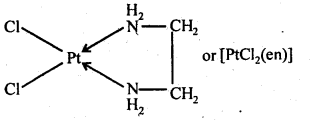
9.27. Discuss briefly giving an example in each case the role of coordination compounds in :
(a) biological systems,
(b) analytical chemistry,
(c) medicinal chemistry, and
(d) extraction/metallurgy of metals.
Ans:
(i) Coordination compounds are of great importance in biological systems. The pigment responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin, the red pigment 1 of blood which acts as oxygen carrier is a coordination compound of iron. Vitamin B12, cyanocobalamine, the anti- pernicious anaemia factor, is a coordination compound of cobalt. Among the other compounds of biological importance with coordinated metal ions are the enzymes like, carboxypeptidase A and carbonic anhydrase (catalysts of biological systems).
(ii) There is growing Interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant/animal systems. Thus, excess of copper and iron are removed by the chelating ligands D-penicillamine and desferrioxime B via the formation of coordination compounds.
EDTA is used in the treatment of lead poisoning. Some coordination compounds of platinum effectively inhibit the growth of tumours. Examples are: ds-platin and related compounds.
(iii) Coordination compounds find use in many qualitative and quantitative chemical analysis. The familiar colour reactions given by metal ions with a number of ligands (especially chelating ligands), as a result of formation of coordination entities, form the basis for their detection and estimation by classical and instrumental methods of analysis. Examples of such reagents include EDTA, DMG (dimethylglyoxime), a-nitroso- β-naphthol, cupron, etc.
(iv) Some important extraction processes of metals, like those of silver and gold, make use of complex formation. Gold, for example, combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN)2]- in aqueous solution. Gold can be separated in metallic form from this solution by the addition of zinc.
9.28. How many ions are produced from the complex Co(NH3)6Cl2 in solution?
(i) 6
(ii) 4
(iii)3
(iv)2
Ans: Coordination number of cobalt = 6
Hence, the complex is [Co (NH3)6] Cl2. It ionizes in the solution as follows :![]()
Thus, three ions are produced. Hence, the correct option is (iii)
9.29. Amongst the following ions? Which one has the highest magnetic moment value:
(i) [Cr(H2O)6]3+
(ii) [Fe(H20)6]2+ (iii) [Zn(H20)6]2+
Ans: The oxidation states are: Cr (III), Fe (II) and Zn (II).
Electronic configuration of Cr3+ = 3d3, unpaired electron = 3
Electronic configuration of Fe2+ = 3d6, unpaired electron = 4
Electronic configuration ofZn2+ = 3d10, unpaired electrons = 0
where V is number of unpaiared electrons Hence, (ii) has highest value of magnetic moment.
9.30. The oxidation number of cobalt in K[Co(CO)4] is
(i)+1
(ii)+3
(iii)-1
(iv)-3
Ans: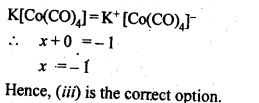
9.31. Amongst the following, the most stable complex is:
(i) [Fe(H2O)6] (ii) [Fe(NH3)6]3+
(iii) [Fe(C2O4)3]3- (iv) [FeCl6]3-
Ans: In each of the given complex, Fe is in + 3 oxidation state. As C2042-is didentate chelating ligand, it forms chelate rings and hence (iii) out of complexes given above is the most stable complex.
9.32. What will be the correct order for the wavelengths of absorption in the visible region for the following:[Ni(NO2)6]4-, [Ni(NH3)6]2+, [Ni(H20)6]2+?
Ans: As metal ion is fixed, the increasing field strengths, i.e., the CFSE values of the ligands from the spectro-chemical series are in the order: H20<NH3< NO2–;
Thus, the energies absorbed for excitation will be in the order: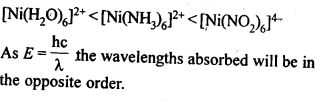
Haloalkanes and Haloarenes
| Section Name | Topic Name |
| 10 | Haloalkanes and Haloarenes |
| 10.1 | Classification |
| 10.2 | Nomenclature |
| 10.3 | Nature of C–X Bond |
| 10.4 | Methods of Preparation of Haloalkanes |
| 10.5 | Preparation of Haloarenes |
| 10.6 | Physical Properties |
| 10.7 | Chemical Reactions |
10.1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcydohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene.
Ans:
10.2. Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans: KI is expected to give HI on reacting with H2SO4 which will convert alcohols (R – OH) to alkyl iodides (R – I). However, H2SO4 is a strong oxidising agent and it oxidises HI formed during the reaction to I2 which does not react with alcohol.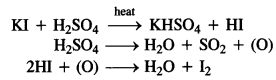
To solve the problem, H2S04 is replaced by phosphoric acid (H3P04) which provides HI for the reaction and does not give I2 as is done by H2S04.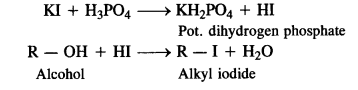
10.3. Write structures of different dihalogen derivatives of propane.
Ans: Four isomers are possible. These are :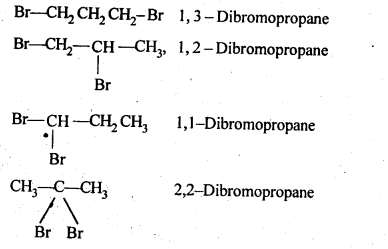
10.4. Among the isomeric alkanes of mdlecular formula C5H12, identify the one that on photochemical chlorination yields
(i) A single monochloride.
(ii) Three isomeric monochlorides.
(iii) Four isomeric monochlorides.
Ans: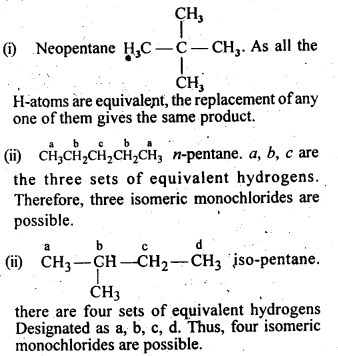
10.5. Draw the structures of major monohalo products in each of the following reactions: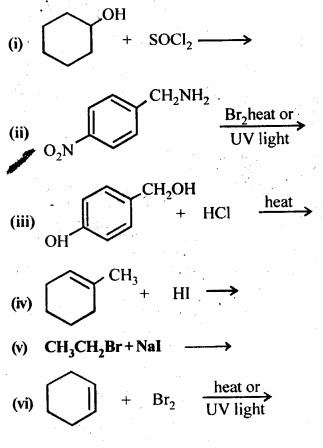
Ans: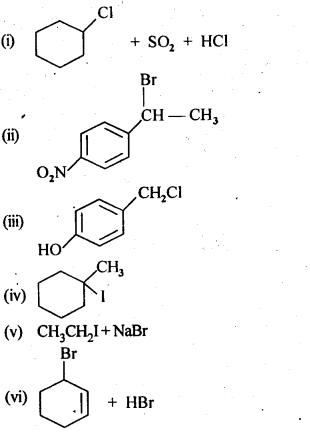
10.6. Arrange each set of compounds in order of increasing boiling points :
(i) Bromomethane, bromoform, chloromethane, dibromomethane
(ii) 1- Chloropropane, isopropylchloride, 1- chlorobutane.
Ans:
(i) The boiling points of organic compounds are linked with the van der Waals’ forces of attraction which depend upon the molecular size. In the present case, all the compounds contain only one carbon atom. The molecular size depends upon size of the halogen atom and also upon the number of halogen atoms present in different molecules. The increasing order of boiling points is :
CH3Cl(chloromethane) < CH3Br (bromomethane) < CH2Br2 (dibromomethane) < CHBr3 (bromoform)
(ii) The same criteria is followed in this case. We all know that the branching of the carbon atom chain decreases the size of the isomer and this decreases its boiling point as compared to straight chain isomer. The increasing order of boiling point is :
(CH3)2CHCl (isopropylchloride or 2-chloropropane) < ClCH2CH2CH3 (1-chloropropane) < ClCH2CH2CH2CH3 (1-chlorobutane)
10.7. Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.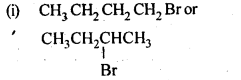
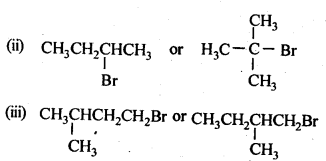
Ans: In SN2 mechanism, reactivity depends upon the steric hindrance around the C-atom carrying the halogen. Lesser the steric hindrance, faster the reaction.
10.8. In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?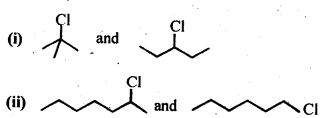
Ans:
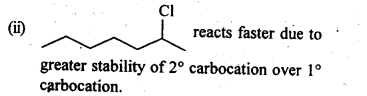
10.9. Identify A, B, C, D, E, R and R1 in the following:
Ans:
10.10. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene. Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.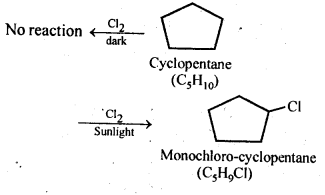
10.1. Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl, or aryl halides:
(i)(CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)CI
(iii) CH3CH2C(CH3)2CH2I
(iv)(CH3)3CCH2CH(Br)C6H5
(v)CH3CH(CH3)CH(Br)CH3
(vi)CH3C(C2H5)2CH2Br
(vii)CH3C(Cl)(C2H5)CH2CH3
(viii)CH3CH=C(CI)CH2CH(CH3)2
(ix)CH3CH=CHC(Br)(CH3)2
(x)P-CIC6H4CH2CH(CH3)2
(xi)m-ClCH2C6H4CH2C(CH3)3
(xii)o-Br -C6H4CH (CH3)CH2CH3
Ans: (i) 2-Chloro-3methylbutane, 2° alkyl halide
(ii) 3-Chloro-4methyl hexane, 2° alkyl halide
(iii) 1 -Iodo-2,2-dimethylbutane, 1 ° alkyl halide
(iv) l-Bromo-3, 3-dimethyl -1-phenylbutane, 2° benzylic halide
(v) 2-Bromo-3-methylbutane, 2° alkyl halide
(vi) 1-Bromo-2-ethyI-2-methylbutane, 1° alkyl halide
(vii)3-Chloro-3-methylpentane, 3° alkyl halide
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide
(ix)4-Bromo-4-methylpent-2-ene, allylic halide
(x)1-Chloro-4-(2-methylpropyl) benzene, aryl halide
(xi)1-Chloromethyl-3- (2,2-dimethylpropyl) benzene, 1 ° benzylic halide.
(xii)1-Bromo-2-(l-methylpropyl) benzene,aryl halide.
10.2. Give the IUPAC names of the following compounds:
(i) CH3CH(CI)CH (Br)CH3 (ii) CHF2CBrCIF (iii) CICH2C=CCH2Br (iv) (CCl3)3CCl
(v)CH3C(p-ClC6H4)2CH(Br)CH3 (vi)(CH3)3CCH=C(CI)C6H4I -p
Ans: (i) 2-Bromo-3-chlorobutane
(ii) 1 JBromo-1 -chloro-1,2,2-trifluoroethane
(iii) l-Bromo-4-chlorobut-2-yne
(iv)2-(Trichloromethyl)-l, 1,1,2,3,3,3- heptachloropropane
(v)2-Bromo-3,3-bis-(4-chlorophenyl) butane
(vi)l-Chloro-l-(4-iodophenyl)-3,3- dimethylbut-l-ene.
10.3. Write the structures of the following organic halogen compounds:
(i)2-ChIoro-3-methylpentane
(ii)p-Bromochlorobenzene
(iii)l-Chloro-4-ethylcyclohexane
(iv)2r (2-Chlorophenyl) -1- iodooctane
(v)2-Bromobutane
(vi)4-tert-Butyl-3-iodoheptane
(vii)1-Bromo-4-sec-butyl-2-methylbenzene
(viii)1,4-Dibromobut-2-ene
Ans:
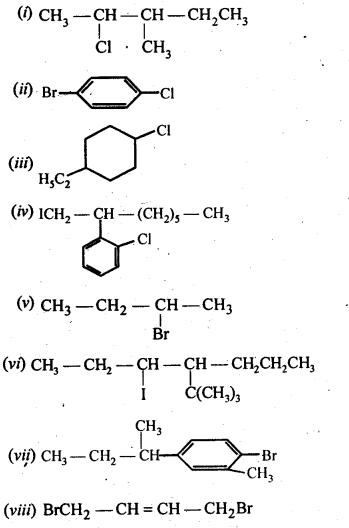
10.4. Which one of the following has the highest dipole moment?
(i)CH3CI2 (ii) CHCl3 (iii) CCI4
Ans: The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:
CCl4 being symmetrical has zero dipole moment. In CHCl3, the resultant of two C – Cl dipole moments is opposed by the resultant of C – H and C – Cl bonds. Since the dipole moment of latter resultant is expected to be smaller than the former, CHCl3 has a finite dipole (1.03 D) moment.
In CH2CI2, the resultant of two C – Cl dipole moments is reinforced by resultant of two C – H dipoles, therefore, CH2CI2 (1 .62 D) has a dipole moment higher than that of CHCl3. Thus, CH2CI2 has highest dipole moment.
10.5. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9CI in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H, 0 can either a cycloalkane or an alkene.
Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
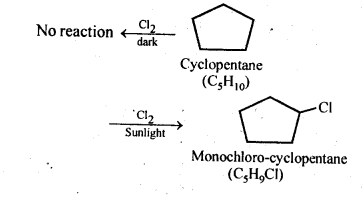
10.6. Write the isomers of the compound having formula C4H9Br.
Ans:
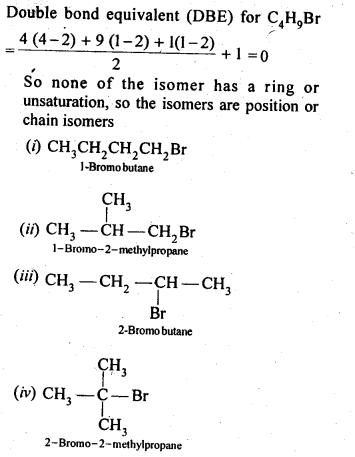
10.7. Write the equations for the preparation of 1-iodoobutanefrom (i)1-butanol (ii)1-chlorobutane (iii) but-l-ene.
Ans:
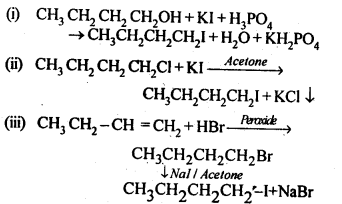
10.8. What are ambident nucleophiles ? Explain with an example.
Ans: Nucleophiles which can attack through two different sites are called ambident nucleophiles. For example, cyanide ion is a resonance hybrid of the following two structures:

It can attack through carbon to form cyanide and through N to form is O cyanide.
10.9. Which compound in each of the following-pairs . will react faster in SN2 reaction with -OH? (i)CH3Br or CH3I
(ii)(CH3)3CCl or CH3Cl
Ans: (i)Since I– ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH– ion.
(ii)On steric grounds, 1° alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3CI will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH– ion.
10.10. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(I) 1-Bromo-l-methylcyclohexane
(ii) 2-Chloro-2-methylbutane.
(iii) 2,2,3-Trimethyl-3-bromopentane.
Ans:
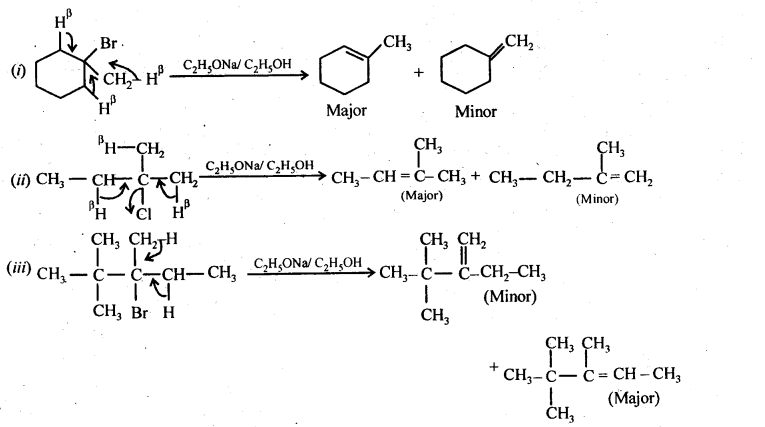
10.11. How will you bring about the following conversions?
(I) Ethanol to but-l-yne.
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-l-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl
Ans:
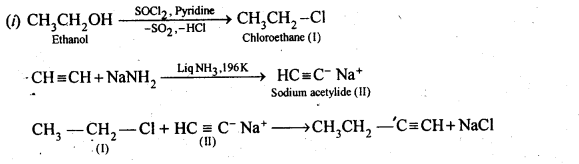
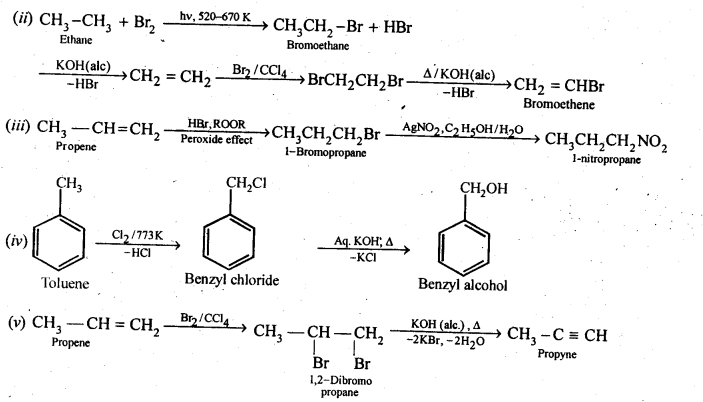
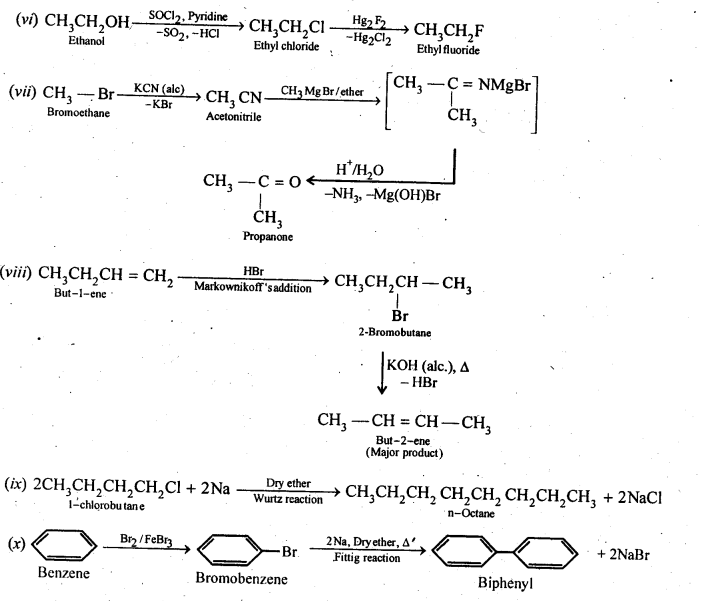
10.12. Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Ans: (i) sp2-hybrid carbon in chlorobenzene is more electronegative than a sp3-hybrid carbon in cyclohexylchloride, due to greater s-character. Thus, C atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
As a result, C – Cl bond in chlorobenzene is less polar than in cyclohexylchloride. Further, due to delocalization of lone pairs of electrons of the Cl atom over the benzene ring, C-Cl bond in chlorobenzene acquires some double bond character while the C – Cl in cyclohexy! chloride is a pure single bond. In other words, C-Cl bond in chlorobenzene is shorter than in cyclohexyl chloride.
Since dipole moment is a product of charge and distance, therefore, chlorobenzene has lower dipole moment than cyclohexylchloride due to lower magnitude of negative charge on the Cl atom and shorter C-Cl distance.
(ii) Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attraction. The molecules of H2O are hold together by H-bonds. Since the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide – alkyl halide molecules and water-water molecules, thefefore, alkyl halides are immiscible (not soluble) in water. Alkyl halide are neither able to form H- bonds with water nor are able to break the H-bounding network of water.
(iii)Grignard reagents are very reactive. They react with moisture present in the apparatus to form alkanes
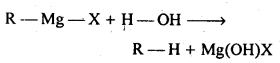
Thus, Grignard reagents must be prepared under anhydrous conditions.
10.13. Give the uses of freon 12, DDT, carbon tetrachloride, and iodoform.
Ans: Iodoform: It was earlier used as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself. Due to its objectionable smell, it has been replaced by other formulations containing iodine.
Carbon tetrachloride:
Uses:
(i)As an industrial solvent for oil, fats, resins etc.and also in dry cleaning.
(ii)CCl4 vapours are highly non-inflammable, thus CCl4 is used as a fire extinguisher under the name pyrene.
(iii)Used in the manufacture of refrigerants and propellants for aerosol cans.
Freons: Freon-12 (CCl2F2) is most common freons in industrial use.
Uses: For aerosol propellants, refrigeration, and air conditioning purposes.
DDT (p -p’ – Dichloro diphenyl – trichloro ethane):
(i)The use of DDT increased enormously on a worldwide basis after World War II, primarily because of its effectiveness against the mosquitoes that spreads malaria and other insects which damages crops.
(ii) However, problems related to extensive use of DDT began to appear in the late 1940 s. Many species of insects developed resistance to DDT, it was also discovered to have a high toxicity towards fishes. DDT is not metabolised very rapidly by animals, instead, it is deposited and stored in the fatty tissues. If the ingestion continues at a steady rate, DDT builds up within the animal’s overtime.
10.14. Write the structure of the major organic product in each of the following reactions:
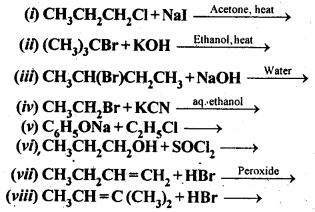
Ans:
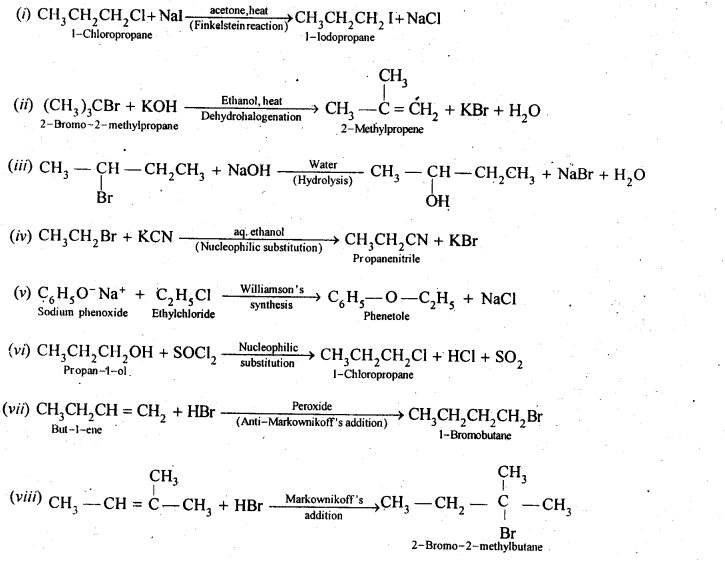
10.15. Write the mechanism of the following reaction:
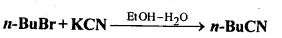
Ans: KCN is a resonance hybrid of the following two contributing structures:
Thus, CN– ion is an ambident nucleophile. Therefore, it can attack the “carbon atom of C-Br bond in n-BuBr either through C or N. Since C – C bond is stronger than C – N bond, therefore, attack occurs through C to form n-butyl cyanide.
10.16. Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methyibutane, 1-Bromopentane, 2-Bromopentane.
(ii) l-Bromo-3-methyIbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane.
(iii) 1-Bromobutane, l-Bromo-2,2-dimethylpropane, l-Bromo-2-methylbutane, l-Bromo-3-methyl butane.
Ans: The SN2 reactions reactivity depends upon steric hindrance. More the steric hindrance slower the reaction.Thus the order of reactivity will be 1°> 2° >3°
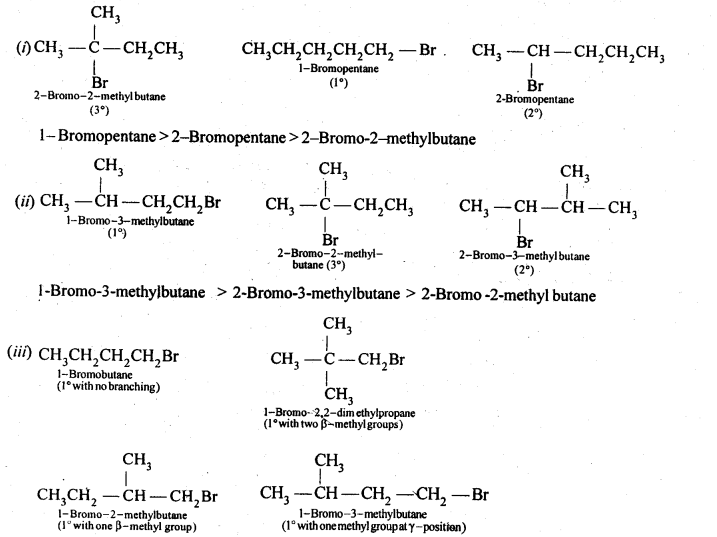
Since in case of 1° alkyl halides steric hindrance increases in the order) n-alkyl halides, alkyl halides with a substituent at any position other than the β-position, one substituent at the β-position, two substituents at the β-position, therefore, the reactivity decreases in the same order. Thus, the reactivity of the given alkyl bromides decreases in the order:
1-Bromobutane > l-Bromo-3-methylbutane > l-Bromo-2-methyjbutane> 1-Bromo-2,2-dimethyl propane.
10.17. Out of C6H5CH2Cl and C6H5CHCIC6H5which is more easily hydrolysed by aqueous KOH.
Ans: C6H5CH2Cl is 10 aryl halide while C6H5CH(CI)C6H5 is a 2° aryl halide. In SN1 reactions, the reactivity depends upon the stability of carbocations.
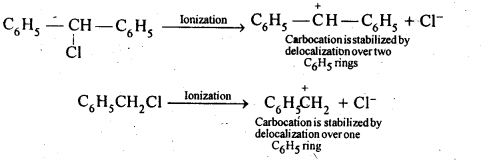
Since the C6H5CHC6H5carbocation is more stable than C6H5CH2 carbocation, therefore,C6H5CHCIC6H5 gets hydrolysed more easily than C6H5CH2Cl under SN1 conditions. However, under SN2 conditions, the reactivity depends on steric hindrance, therefore, under SN2 conditions,C6H5CH2Cl gets hydrolysed more easily than C6H5CHClC6H5.
10.18. p-dichlorobenzene has higher m.p. and lesser solubility than those of o-and m-isomers. Discuss. (C.B.S.E. Delhi 2013)
Ans: The three isomers are position isomers which differ in the relative positions of the chlorine atoms in the ring :
As we know, p-isomer is more symmetrical as compared to the other isomers. This means that in the crystal lattice, molecules of the p-isomers are more closely packed as compared to the other isomers. As a result, it has a higher melting point and lower solubility as compared to ortho and meta isomers.
Haloarenes are less polar than haloalkanes and are insoluble in water. This is because of lack of hydrogen bonding. As a result, the attractive forces in haloarenes—water system remain less than the attractive forces in H20 molecules which are hydrogen bonded. Haloarenes are soluble in organic solvents of low polarity such as benzene, ether, chloroform, carbon tetrachioride etc.
10.19. How the following conversions can be carried out:
(i) Propene to propan-l-ol (ii) Ethanol to but-l-yne
(iii) l-Bromopropane to 2-bromopropane (iv) Toluene to benzyl alcohol
(v)Benzene to 4-bromonitrobenzene (vi) Benzyl alcohol to 2-phenylethanoic acid
(vii)Ethanol to propanenitrile (viii) Aniline to chlorobenzene
(ix)2-Chlorobutane to 3,4-dimethylhexane (x) 2-Methyl-1 -propene to 2-chk>ro-2-methylpropane.
(xi)Ethyl chloride to propanoic acid (xii) But-1-ene to n-butyliodide
(xiii)2-Chlropropane to 1-propanol (xiv) Isopropyl alcohol to iodoform
(xv)Chlorobenzene to p-nitrophenol (xvi) 2-Bromopropane to 1-bromopropane
(xvii)Chloroethane to butane , (xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide (xx) Aniline to phenylisocyanide
Ans:
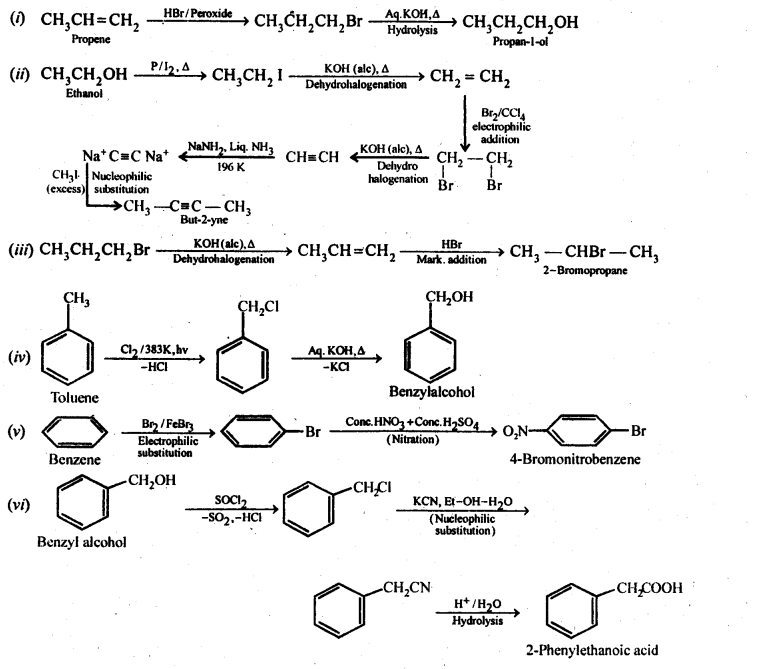
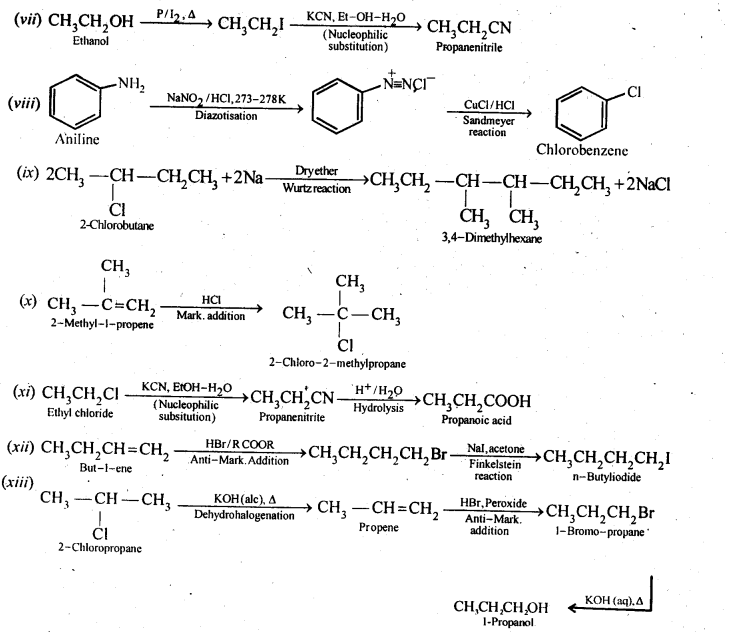
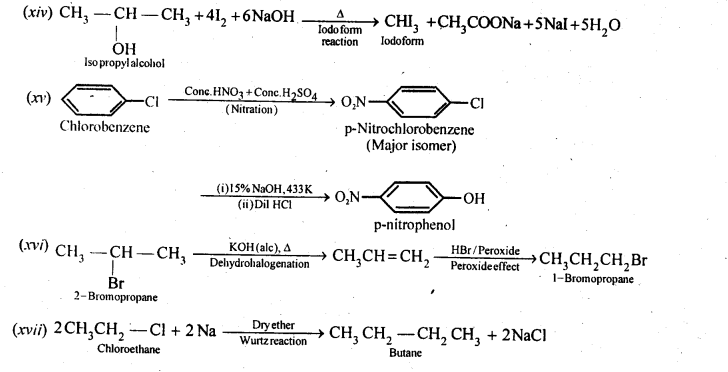
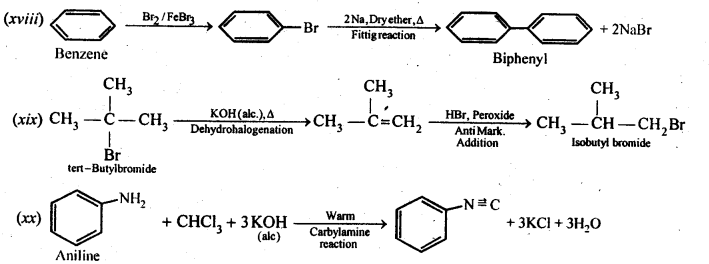
10.20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH, alkenes are major products. Explain. (Pb. Board 2009, Haryana Board 2013)
Answer:
In aqueous medium i.e., water, KOH will be completely dissociated to give OH– ions. They being a strong nucleophile, will bring about the substitution of alkyl halides to form alcohols. At the same time, the OH” ions will be highly hydrated also. They will not be able to abstract a proton (H+) from the p-carbon atom to form alkenes. In other words, in aqueous medium, OH– ions will behave as weak base and elimination leading to alkenes will not be feasible.
In alcoholic KOH, the solution will also contain ethoxide ions (C2H5O–) in addition to OH– ions. They being a stronger base than OH– ions, will abstract a H+ ion from the β-carbon atom giving alkene as the product as a result of dehydrohalogenation.
10.21. Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b) Compound (b) is reacted, with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it give compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans: (i) There are two primary alkyl halides having the molecular formula, C4H9Br.
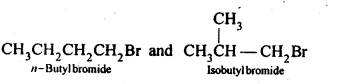
(ii) Since compound (a) when reacted with Na metal gave a compound (d) with molecular formula C8H18 which was different from die compound obtained when n-butyl bromide was reacted with Na metal, therefore, (a) must be isobutyl bromide and compound (d) must be 2,3-dimethylhexane.
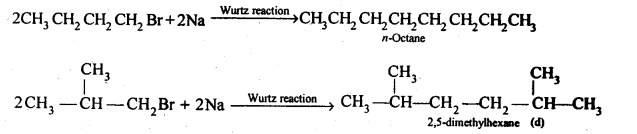
(iii) If compound (a) is isobutyl bromide, than the compound (b) which it gives on treatment with alcoholic KOH must be 2-methyl-1-propane.
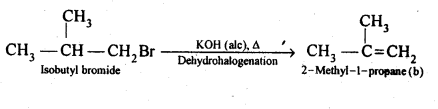
(iv) The compound (b) on treatment with HBr gives compound (c) in accordance with Markownikoff rule. Therefore, compound (c) is tert-butyl bromide which is an isomer of compound (a) ,i.e., isobutyl ‘ bromide.
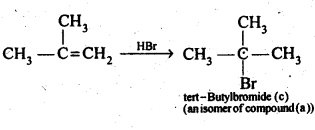
Thus
(a)is isobutyl bromide,
(b)is 2-methyl-1 -propane,
(c)is tert-butylbromide, and
(d)is 2,5-dimethylhexane.
10.22. What happens when .
(i) n-butyi chloride is treated with alcoholic KOH.
(ii) bromobenzene is treated with Mg in the presence of dry ether.
(iii) chlorobenzene is subjected to hydrolysis.
(iv) ethyl chloride is treated with aqueous. KOH.
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN.
Ans:
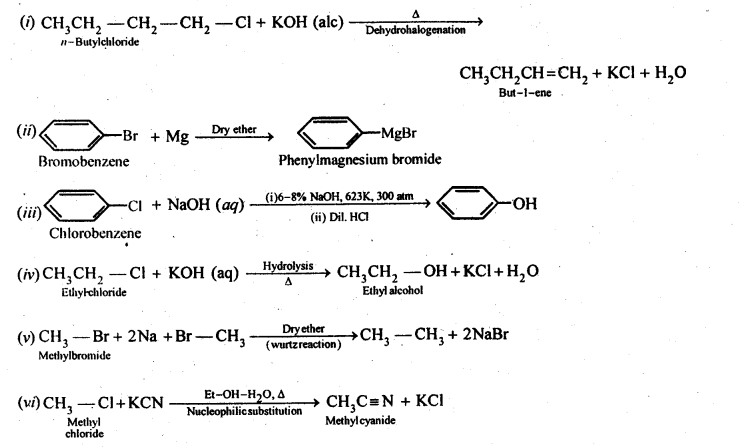
10.1. Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl, or aryl halides:
(i)(CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)CI
(iii) CH3CH2C(CH3)2CH2I
(iv)(CH3)3CCH2CH(Br)C6H5
(v)CH3CH(CH3)CH(Br)CH3
(vi)CH3C(C2H5)2CH2Br
(vii)CH3C(Cl)(C2H5)CH2CH3
(viii)CH3CH=C(CI)CH2CH(CH3)2
(ix)CH3CH=CHC(Br)(CH3)2
(x)P-CIC6H4CH2CH(CH3)2
(xi)m-ClCH2C6H4CH2C(CH3)3
(xii)o-Br -C6H4CH (CH3)CH2CH3
Ans: (i) 2-Chloro-3methylbutane, 2° alkyl halide
(ii) 3-Chloro-4methyl hexane, 2° alkyl halide
(iii) 1 -Iodo-2,2-dimethylbutane, 1 ° alkyl halide
(iv) l-Bromo-3, 3-dimethyl -1-phenylbutane, 2° benzylic halide
(v) 2-Bromo-3-methylbutane, 2° alkyl halide
(vi) 1-Bromo-2-ethyI-2-methylbutane, 1° alkyl halide
(vii)3-Chloro-3-methylpentane, 3° alkyl halide
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide
(ix)4-Bromo-4-methylpent-2-ene, allylic halide
(x)1-Chloro-4-(2-methylpropyl) benzene, aryl halide
(xi)1-Chloromethyl-3- (2,2-dimethylpropyl) benzene, 1 ° benzylic halide.
(xii)1-Bromo-2-(l-methylpropyl) benzene,aryl halide.
10.2. Give the IUPAC names of the following compounds:
(i) CH3CH(CI)CH (Br)CH3 (ii) CHF2CBrCIF (iii) CICH2C=CCH2Br (iv) (CCl3)3CCl
(v)CH3C(p-ClC6H4)2CH(Br)CH3 (vi)(CH3)3CCH=C(CI)C6H4I -p
Ans: (i) 2-Bromo-3-chlorobutane
(ii) 1 JBromo-1 -chloro-1,2,2-trifluoroethane
(iii) l-Bromo-4-chlorobut-2-yne
(iv)2-(Trichloromethyl)-l, 1,1,2,3,3,3- heptachloropropane
(v)2-Bromo-3,3-bis-(4-chlorophenyl) butane
(vi)l-Chloro-l-(4-iodophenyl)-3,3- dimethylbut-l-ene.
10.3. Write the structures of the following organic halogen compounds:
(i)2-ChIoro-3-methylpentane
(ii)p-Bromochlorobenzene
(iii)l-Chloro-4-ethylcyclohexane
(iv)2r (2-Chlorophenyl) -1- iodooctane
(v)2-Bromobutane
(vi)4-tert-Butyl-3-iodoheptane
(vii)1-Bromo-4-sec-butyl-2-methylbenzene
(viii)1,4-Dibromobut-2-ene
Ans:
10.4. Which one of the following has the highest dipole moment?
(i)CH3CI2 (ii) CHCl3 (iii) CCI4
Ans: The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:
CCl4 being symmetrical has zero dipole moment. In CHCl3, the resultant of two C – Cl dipole moments is opposed by the resultant of C – H and C – Cl bonds. Since the dipole moment of latter resultant is expected to be smaller than the former, CHCl3 has a finite dipole (1.03 D) moment.
In CH2CI2, the resultant of two C – Cl dipole moments is reinforced by resultant of two C – H dipoles, therefore, CH2CI2 (1 .62 D) has a dipole moment higher than that of CHCl3. Thus, CH2CI2 has highest dipole moment.
10.5. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9CI in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H, 0 can either a cycloalkane or an alkene.
Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
10.6. Write the isomers of the compound having formula C4H9Br.
Ans:
10.7. Write the equations for the preparation of 1-iodoobutanefrom (i)1-butanol (ii)1-chlorobutane (iii) but-l-ene.
Ans:
10.8. What are ambident nucleophiles ? Explain with an example.
Ans: Nucleophiles which can attack through two different sites are called ambident nucleophiles. For example, cyanide ion is a resonance hybrid of the following two structures:![]()
It can attack through carbon to form cyanide and through N to form is O cyanide.
10.9. Which compound in each of the following-pairs . will react faster in SN2 reaction with -OH? (i)CH3Br or CH3I
(ii)(CH3)3CCl or CH3Cl
Ans: (i)Since I– ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH– ion.
(ii)On steric grounds, 1° alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3CI will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH– ion.
10.10. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(I) 1-Bromo-l-methylcyclohexane
(ii) 2-Chloro-2-methylbutane.
(iii) 2,2,3-Trimethyl-3-bromopentane.
Ans:
10.11. How will you bring about the following conversions?
(I) Ethanol to but-l-yne.
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-l-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl
Ans:


10.12. Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Ans: (i) sp2-hybrid carbon in chlorobenzene is more electronegative than a sp3-hybrid carbon in cyclohexylchloride, due to greater s-character. Thus, C atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
As a result, C – Cl bond in chlorobenzene is less polar than in cyclohexylchloride. Further, due to delocalization of lone pairs of electrons of the Cl atom over the benzene ring, C-Cl bond in chlorobenzene acquires some double bond character while the C – Cl in cyclohexy! chloride is a pure single bond. In other words, C-Cl bond in chlorobenzene is shorter than in cyclohexyl chloride.
Since dipole moment is a product of charge and distance, therefore, chlorobenzene has lower dipole moment than cyclohexylchloride due to lower magnitude of negative charge on the Cl atom and shorter C-Cl distance.
(ii) Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attraction. The molecules of H2O are hold together by H-bonds. Since the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide – alkyl halide molecules and water-water molecules, thefefore, alkyl halides are immiscible (not soluble) in water. Alkyl halide are neither able to form H- bonds with water nor are able to break the H-bounding network of water.
(iii)Grignard reagents are very reactive. They react with moisture present in the apparatus to form alkanes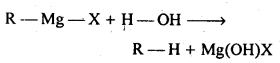
Thus, Grignard reagents must be prepared under anhydrous conditions.
10.13. Give the uses of freon 12, DDT, carbon tetrachloride, and iodoform.
Ans: Iodoform: It was earlier used as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself. Due to its objectionable smell, it has been replaced by other formulations containing iodine.
Carbon tetrachloride:
Uses:
(i)As an industrial solvent for oil, fats, resins etc.and also in dry cleaning.
(ii)CCl4 vapours are highly non-inflammable, thus CCl4 is used as a fire extinguisher under the name pyrene.
(iii)Used in the manufacture of refrigerants and propellants for aerosol cans.
Freons: Freon-12 (CCl2F2) is most common freons in industrial use.
Uses: For aerosol propellants, refrigeration, and air conditioning purposes.
DDT (p -p’ – Dichloro diphenyl – trichloro ethane):
(i)The use of DDT increased enormously on a worldwide basis after World War II, primarily because of its effectiveness against the mosquitoes that spreads malaria and other insects which damages crops.
(ii) However, problems related to extensive use of DDT began to appear in the late 1940 s. Many species of insects developed resistance to DDT, it was also discovered to have a high toxicity towards fishes. DDT is not metabolised very rapidly by animals, instead, it is deposited and stored in the fatty tissues. If the ingestion continues at a steady rate, DDT builds up within the animal’s overtime.
10.14. Write the structure of the major organic product in each of the following reactions:
Ans:
10.15. Write the mechanism of the following reaction:![]()
Ans: KCN is a resonance hybrid of the following two contributing structures:![]()
Thus, CN– ion is an ambident nucleophile. Therefore, it can attack the “carbon atom of C-Br bond in n-BuBr either through C or N. Since C – C bond is stronger than C – N bond, therefore, attack occurs through C to form n-butyl cyanide.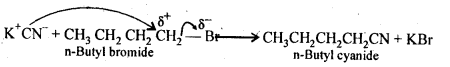
10.16. Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methyibutane, 1-Bromopentane, 2-Bromopentane.
(ii) l-Bromo-3-methyIbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane.
(iii) 1-Bromobutane, l-Bromo-2,2-dimethylpropane, l-Bromo-2-methylbutane, l-Bromo-3-methyl butane.
Ans: The SN2 reactions reactivity depends upon steric hindrance. More the steric hindrance slower the reaction.Thus the order of reactivity will be 1°> 2° >3°
Since in case of 1° alkyl halides steric hindrance increases in the order) n-alkyl halides, alkyl halides with a substituent at any position other than the β-position, one substituent at the β-position, two substituents at the β-position, therefore, the reactivity decreases in the same order. Thus, the reactivity of the given alkyl bromides decreases in the order:
1-Bromobutane > l-Bromo-3-methylbutane > l-Bromo-2-methyjbutane> 1-Bromo-2,2-dimethyl propane.
10.17. Out of C6H5CH2Cl and C6H5CHCIC6H5which is more easily hydrolysed by aqueous KOH.
Ans: C6H5CH2Cl is 10 aryl halide while C6H5CH(CI)C6H5 is a 2° aryl halide. In SN1 reactions, the reactivity depends upon the stability of carbocations.
Since the C6H5CHC6H5carbocation is more stable than C6H5CH2 carbocation, therefore,C6H5CHCIC6H5 gets hydrolysed more easily than C6H5CH2Cl under SN1 conditions. However, under SN2 conditions, the reactivity depends on steric hindrance, therefore, under SN2 conditions,C6H5CH2Cl gets hydrolysed more easily than C6H5CHClC6H5.
10.18. p-dichlorobenzene has higher m.p. and lesser solubility than those of o-and m-isomers. Discuss. (C.B.S.E. Delhi 2013)
Ans: The three isomers are position isomers which differ in the relative positions of the chlorine atoms in the ring :
As we know, p-isomer is more symmetrical as compared to the other isomers. This means that in the crystal lattice, molecules of the p-isomers are more closely packed as compared to the other isomers. As a result, it has a higher melting point and lower solubility as compared to ortho and meta isomers.
Haloarenes are less polar than haloalkanes and are insoluble in water. This is because of lack of hydrogen bonding. As a result, the attractive forces in haloarenes—water system remain less than the attractive forces in H20 molecules which are hydrogen bonded. Haloarenes are soluble in organic solvents of low polarity such as benzene, ether, chloroform, carbon tetrachioride etc.
10.19. How the following conversions can be carried out:
(i) Propene to propan-l-ol (ii) Ethanol to but-l-yne
(iii) l-Bromopropane to 2-bromopropane (iv) Toluene to benzyl alcohol
(v)Benzene to 4-bromonitrobenzene (vi) Benzyl alcohol to 2-phenylethanoic acid
(vii)Ethanol to propanenitrile (viii) Aniline to chlorobenzene
(ix)2-Chlorobutane to 3,4-dimethylhexane (x) 2-Methyl-1 -propene to 2-chk>ro-2-methylpropane.
(xi)Ethyl chloride to propanoic acid (xii) But-1-ene to n-butyliodide
(xiii)2-Chlropropane to 1-propanol (xiv) Isopropyl alcohol to iodoform
(xv)Chlorobenzene to p-nitrophenol (xvi) 2-Bromopropane to 1-bromopropane
(xvii)Chloroethane to butane , (xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide (xx) Aniline to phenylisocyanide
Ans:



10.20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH, alkenes are major products. Explain. (Pb. Board 2009, Haryana Board 2013)
Answer:
In aqueous medium i.e., water, KOH will be completely dissociated to give OH– ions. They being a strong nucleophile, will bring about the substitution of alkyl halides to form alcohols. At the same time, the OH” ions will be highly hydrated also. They will not be able to abstract a proton (H+) from the p-carbon atom to form alkenes. In other words, in aqueous medium, OH– ions will behave as weak base and elimination leading to alkenes will not be feasible.
In alcoholic KOH, the solution will also contain ethoxide ions (C2H5O–) in addition to OH– ions. They being a stronger base than OH– ions, will abstract a H+ ion from the β-carbon atom giving alkene as the product as a result of dehydrohalogenation.
10.21. Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b) Compound (b) is reacted, with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it give compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans: (i) There are two primary alkyl halides having the molecular formula, C4H9Br.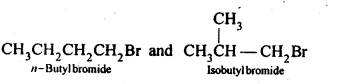
(ii) Since compound (a) when reacted with Na metal gave a compound (d) with molecular formula C8H18 which was different from die compound obtained when n-butyl bromide was reacted with Na metal, therefore, (a) must be isobutyl bromide and compound (d) must be 2,3-dimethylhexane.
(iii) If compound (a) is isobutyl bromide, than the compound (b) which it gives on treatment with alcoholic KOH must be 2-methyl-1-propane.
(iv) The compound (b) on treatment with HBr gives compound (c) in accordance with Markownikoff rule. Therefore, compound (c) is tert-butyl bromide which is an isomer of compound (a) ,i.e., isobutyl ‘ bromide.
Thus
(a)is isobutyl bromide,
(b)is 2-methyl-1 -propane,
(c)is tert-butylbromide, and
(d)is 2,5-dimethylhexane.
10.22. What happens when .
(i) n-butyi chloride is treated with alcoholic KOH.
(ii) bromobenzene is treated with Mg in the presence of dry ether.
(iii) chlorobenzene is subjected to hydrolysis.
(iv) ethyl chloride is treated with aqueous. KOH.
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN.
Ans:
Class 12 Chemistry Chapter 11 Alcohols Phenols and Ether
by phani
by phani
NCERT Solutions For Class 12 Chemistry Chapter 11 Alcohols Phenols and Ether
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols Phenols and Ether:
Section Name
Topic Name
11
Alcohols, Phenols and Ethers
11.1
Classification
11.2
Nomenclature
11.3
Structures of Functional Groups
11.4
Alcohols and Phenols
11.5
Some Commercially Important Alcohols
11.6
Ethers
NCERT SolutionsCBSE Sample Papers ChemistryClass 12 Chemistry
NCERT TEXTBOOK QUESTIONS SOLVED
11.1. Classify the following as primary, secondary and tertiary alcohols.
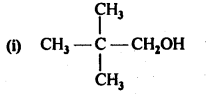
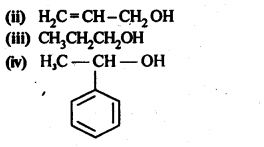
Ans: Primary alcohols: (i), (ii), (iii)
Secondary alcohols: (iv), (v)
Tertiary alcohols: (vi)
11.2. Identify aliylic alcohols in the above examples.
Ans: (ii) and (iv) i.e. H2C=CH – CH2OH and
11.3. Name the following compounds according to IUPAC system.
Ans:
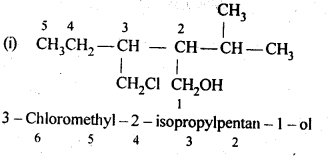
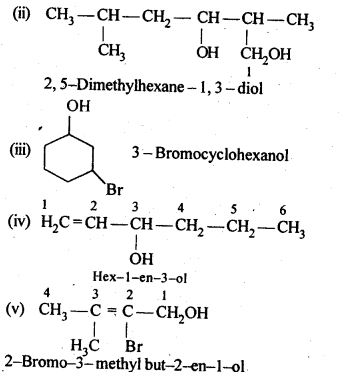
11.4. Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal ?
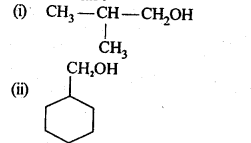
Ans:
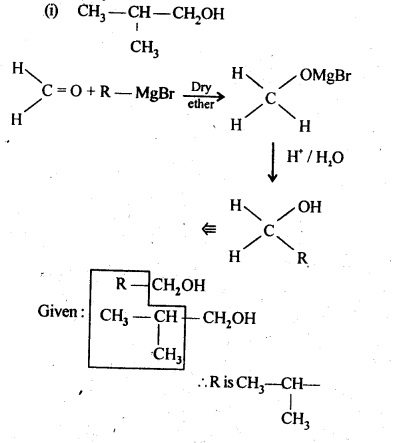
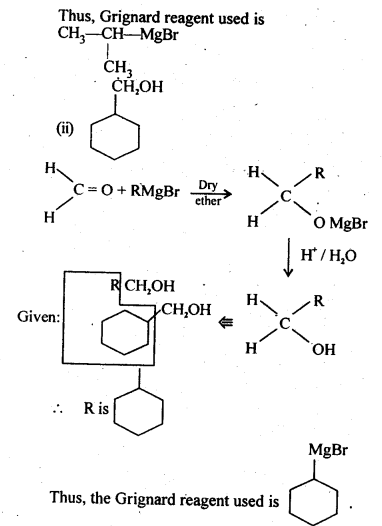
11.5. Write structures of the products of the following reactions:
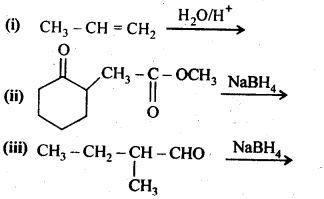
Ans:
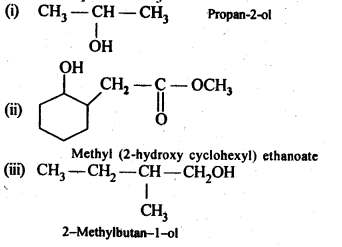
11.6. Give structures of the products you would expect when each of the following alcohol reacts with (a)HCl-ZnCl2 (b)HBrand (c) SOCl2
(i)Butan-1-ol
(ii)2-Methylbutan-2-ol
Ans:
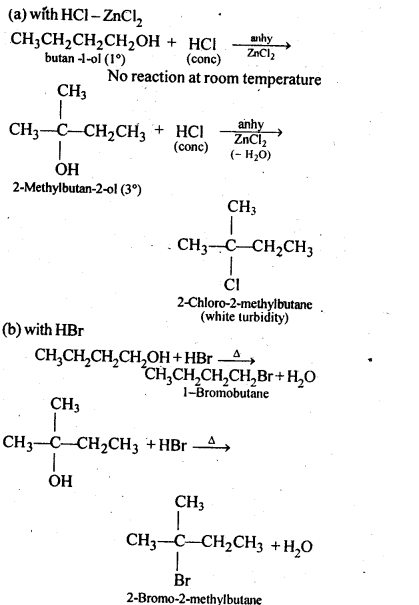
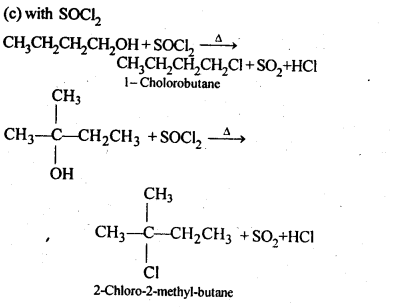
11.7. Predict the major product of acid catalysed dehydration of
(i) 1-nicthylcyclohcxanoland
(ii) butan-1-ol
Ans:
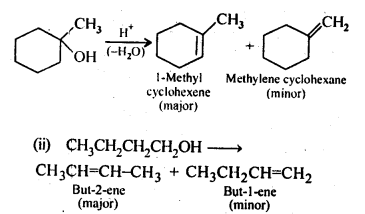
11.8. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Ans:
The resonance structures of o-and p- nitrophenoxide ions and phenoxide ion are given below:
11.9; Write the equations involved in the following reactions:
(i) Reimer-Tiemann reaction
(ii) Kolbe’s reaction
Ans: (i) Reimer-Tiemann reaction
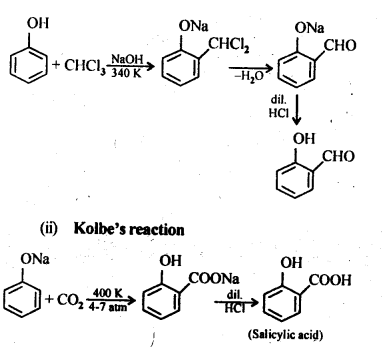
11.10. Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Ans: In Williamsons’s synthesis, the alkyl halide should be primary. Thus, the alkyl halide should be derived from ethanol and the alkoxide ion from 3-methylpentan-2-ol. The synthesis is as follows
11.11. Which of the following is an appropriate set of reactants for the preparation of l-methoxy-4- nitrobenzene and why?
Ans:
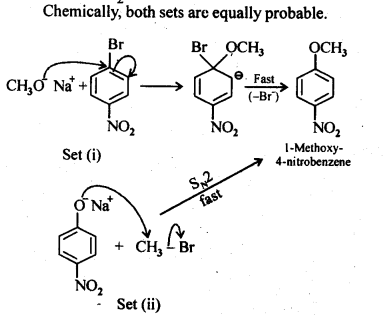
11.12. Predict the products of the following reactions:
Ans:
NCERT EXERCISES
11.1. Write IUPAC names of the following compounds:
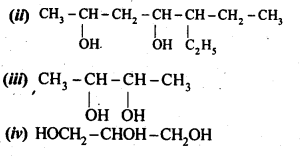
Ans: (i) 2,2,4-Trimethylpentan-3-ol
(ii) 5-Ethylheptane-2,4-dioI
(iii) Butane-2,3-diol
(iv) Propane-1,2,3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2,5-DimethylphenoI
(viii) 2,6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane
11.2. Write structures of the compounds whose IUPAC names are as follows:
(i)2-Methylbutan-2-ol
(ii)l-Phcnylpropan-2-ol
(iii)3,5-DimethyIhexane-l,3,5-triol
(iv)2,3-Dicthylphenol
(v)1-Ethoxypropane
(vi)2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpcntan-3-ol
(ix)Cyclopcnt-3-en-l-ol
(x)4-ChIoro-3-ethylbutan-l-ol
Ans:
11. 3. (a) Draw the structural formulas and write IUPAC names of all the isomeric alkanols with the molecular formula CsH12O
(b) Classify the isomers of alcohols given in part (a) as primary, secondary and tertiary alcohols.
Ans:
(a) The molecular formula C5H120 represents eight isomeric alkanols. These are :
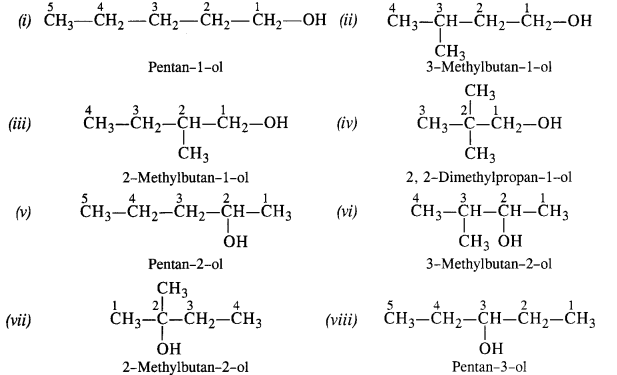
(b) Primary: (i), (ii), (iii), (iv) ; Secondary :(v), (vi), (viii) ; Tertiary : (vii)
11.4. Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Ans: The molecules of butane are held together by weak van der Waal’s forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.
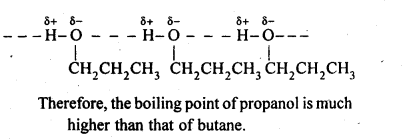
11.5. Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Ans: Alcohols can form hydrogen bonds with water and by breaking the hydrogen bonds already existing between water molecules. Therefore, they are soluble in water.
On die other hand, hydrocarbons cannot from hydrogen bonds with water and hence are insoluble in water.
11.6. What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Ans: The addition of diborane to alkenes to form trialkyl boranes followed by their oxidation with alkaline hydrogen peroxide to form alcohols is called hydroboration-oxidation. For example,
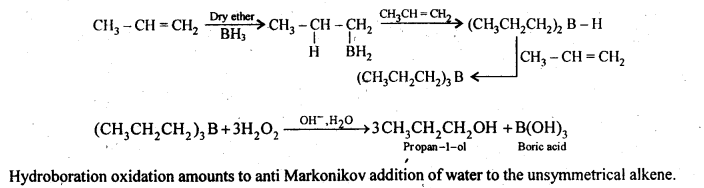
11.7. Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Ans: The three isomers are:
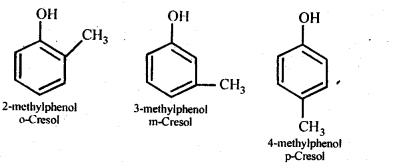
11.8. While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Ans: 0-N itrophenol is steam volatile due to chelation (intramolecular H – bonding) and hence can be separated by steam distillation from/Miitrophenol which is hot steam volatile because of intermolecular H-bonding.
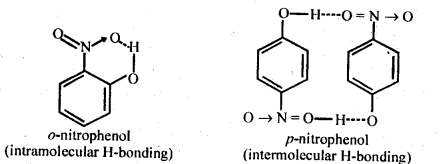
11.9. Give the equations of the reaction for the preparation of phenol from cumene.
Ans: This process has a great industrial importance because it gives the preparation of two very useful compounds i.e. phenol and acetone. The raw materials are benzene and propene and it initially proceeds by Friedel Crafts alkylation of benzene.
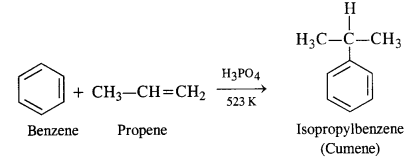
Oxygen is bubbled through the above solution to form cumene hydroperoxide which is decomposed with aqueous acid
solution to form phenol and acetone as follows:
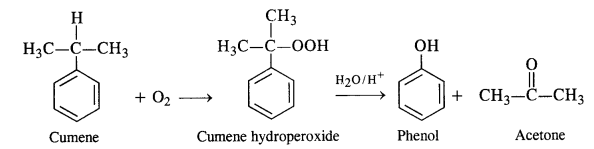
11.10. Write chemical reaction for the preparation of phenol from chlorobenzene.
Ans:
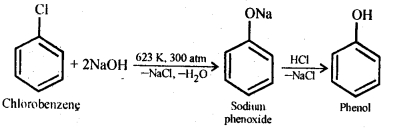
11.11. Write the mechanism of hydration of ethene to yield ethanol.
Ans: Direct addition of H20 to ethene in presence of an acid does not occur. Indirectly, ethene is first passed through concentrated H2S04, when ethyl hydrogen sulphate is formed.
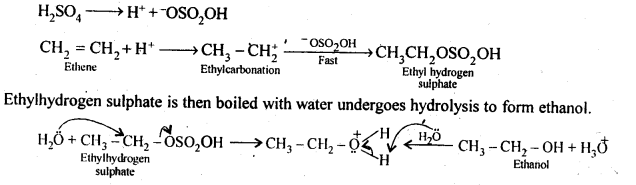
11.12. You are given benzene, cone. H2S04and NaOH. Write the equations for the preparation of phenol using these reagents.
Ans:
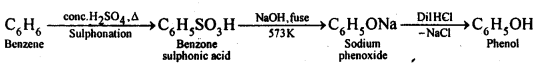
11.13. Show how will you synthesise
(i) 1 -phenylethanol from a suitable alkene.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) Pentan-l-ol using a suitable alkyl halide?
Ans:
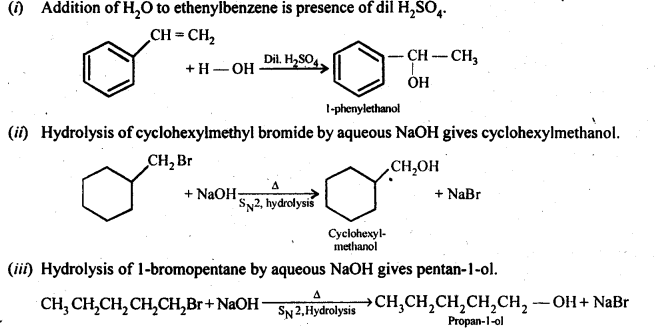
11.14. Give two reactions that show the acidic nature of phenol. Compare its acidity with that of ethanol.
Ans: The reactions showing acidic nature of phenol are:
(a) Reaction with sodium: Phenol reacts with active metals like sodium to liberate H, gas.
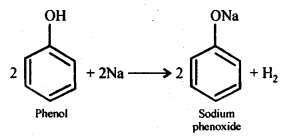
(b) Reaction with NaOH: Phenol dissolves in NaOH to form sodium phenoxide and water.
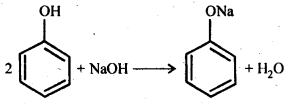
Phenol is more acidic than ethanol. This is due to the reason that phenoxide ion left after the loss of a proton from phenol is stabilized by resonance, while ethoxide ion left after less of a proton from ethanol, is not.
11.15. Explain why is orthonitrophenol more acidic than orthomethoxyphenol?
Ans: Nitro (NO2) group is an electron withdrawing group while methoxy (OCH3) group is electron releasing in nature. The release of H+ ion is therefore, easier from o-nitrophenol while it is quite difficult from o-methoxyphenol. Apart form that, o-nitrophenoxide ion is stabilised due to resonance o-nitrophenol is steam volatile while p-nitrophenol is not. This is on account of intramolecular hydrogen bonding in the molecules of o-nitrophenol. As a result, its boiling point is less than that of p-nitrophenol in which the molecules are linked by intermolecular hydrogen bonding.
It is interesting to note that in the substituted phenols, the nature and position of the substituent influences the boiling point of phenol.
For example: .o-nitrophenol is steam volatile while p-nitrophenol is not. This is supported by the fact that the boiling point temperature of o-nitrophenol (100°C) is less than that of p-nitrophenol, (279°C). In o-nitrophenol, there is intramolecular hydrogen bonding in OH and NO2 groups placed in a adjacent positions. However, these are linked by intermolecular hydrogen bonding in the p-isomers. It is quite obvious that extra energy is needed to the cleave the hydrogen bonds in the p-isomer. Consequently, its boiling point is more.
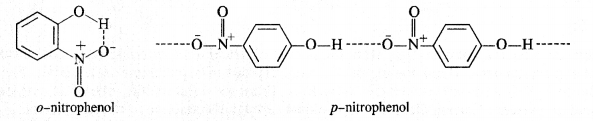
o-nnrophenol with lower boiling point is steam volatile while p-nitrophenol is not. This helps in the separation of the two isomers present in the liquid mixture. On passing steam, o-nitropbenol volatilises and its vapours rise alongwith steam and after condensation, collect in the receiver p-nitrophenol is left behind in the distillation flask. e-nkrophenol p-nnrophenol.
On the contrary, o-methoxyphenoxide is destabilised since the electron density on the negatively charged oxygen tends to increase due to the electron releasing tendency of the methoxy(OCH3) group.
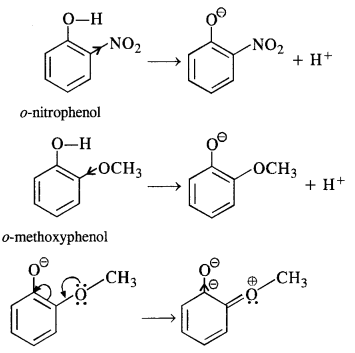
In the light of the above discussion, we may conclude that o-nitrophenol is a stronger acid (pKa = 7-23) than o-methoxyphenl (pKa = 9.98)
11.16. Explain how does the – OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans: Phenol may be regarded as a resonance hybrid of structures I-V, shown below.
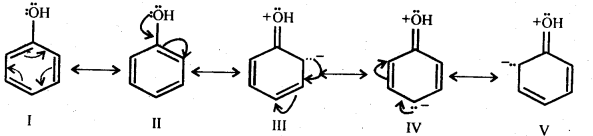
As a result of +R effect of the -OH group, the electron density in the benzene ring increases thereby facilitating the attack of an electrophile. In other words, presence of -OH group, activates the benzene ring towards electrophilic substitution reactions. Further, since the electron density is relatively higher at the two o-and one p-position, therefore electrophilic substitution occurs mainly at o-and p-positions.
11.17. Give equations of the following reactions:
(i) Oxidation of propan-l-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 acid with phenol
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Ans:
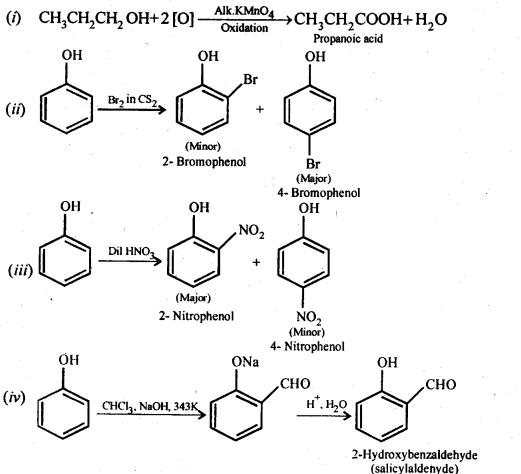
11.18. Explain the following with an example
(i) Kolbe’s reaction
(ii) Reimer – Tiemann reaction –
(iii) Williamson ether synthesis
(iv) Unsymmetrical ether
Ans: (i) Kolbe’s reaction: Sodium phenoxide when heated with C02 at 400K under a pressure of 4-7 atmospheres followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the major product along with a small amount of 4-hydroxybenzoic acid.This reaction is called Kolbe’s reaction.
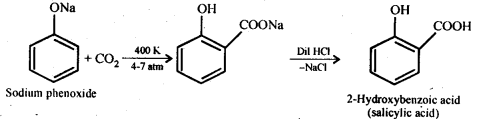
(ii) Reimer-Tiemann reaction: Treatment of phenol with CHC13 in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicyialdehyde) as the major product. This reaction is called Reimer-Tiemann reaction.
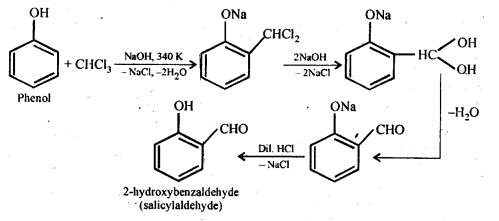
(iii) Williamson’s ether synthesis: It involves the treatment of an alkyl halide with a suitable sodium alkoxide to obtain ethers. The sodium alkoxide needed for the purpose is prepared by the action of sodium on a suitable alcohol. In this reaction alkyl halide should primary. Secondary and tertiary halides will predominantly give an alkene.

(iv) Unsymmetrical ether: If the alkyl or aryl groups attached to the oxygen atom are different, ethers are called unsymmetrical ethers. For example, ethyl methyl ether, methyl phenyl ether, 4-chlorophenyl- 4-nitrophenyl ether, etc.
11.19. Write the mechanism of acid dehydration of ethanol to yield ethene.
Ans: The mechanism of dehydration of alcohols to form alkenes occur by the following three steps:
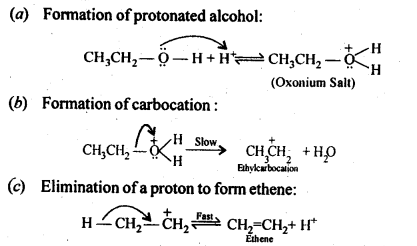
11.20. How are the following conversions carried out?
(i) Propane → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl mag. chloride → Propan-1-ol
(iv) Methyl mag. bromide → 2-Methylpropan-2-ol.
Ans:
11.21. Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Brominationofphenolto2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-oI to propene.
(vi) Butan-2-one to butan-2-oL .
Ans: (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
11.22. Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Ans: Ethanol undergoes intermolecular H-bonding due to the presence of a hydrogen atom attached to the electronegative oxygen atom. As a result, ethanol exists as associated molecules.
Consequently, a large amount of energy is required to break these hydrogen bonds. Therefore, the boiling point of ethanol is higher than that of methoxymethane which does not form H-bonds.
11.23. Give IUPAC names of the following ethers.
Ans: (i)1-Ethoxy-2-methylpropane
(ii) 2-Chlorlo-l-methoxy ethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 1 -Ethoxy-4 -4 – dimethyl cyclohexane
(vi)Ethoxybenzene
11,24. Write the names of the reagents and equations for the preparation of the following ethers by Williamson’s synthesis :
(i) 1-Propoxypropane
(ii) 2-Methoxy-2-methylpropane
(iii) Ethoxybenzene
(iv) Methoxyethane.
Ans:
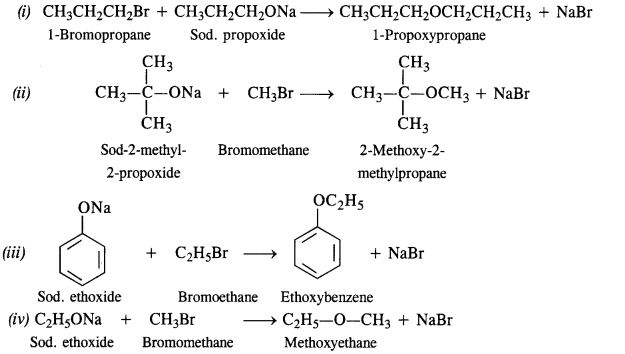
11.25. Illustrate with examples the limitations of Willamson synthesis for the preparation of certain types of ethers.
Ans: Williamson’s synthesis is a versatile method for the synthesis of both symmetrical and unsymmetrical ethers. However, for the synthesis of unsymmetrical ethers, a proper choice of reactants is necessary. Since Williamson’s synthesis occurs by SN2 mechanism and primary alkyl halides are most reactive in Sn2 reaction, therefore, best yields of unsymmetrical ethers are obtained when the alkyl halides are primary and the alkoxide may be primary, secondary or tertiary. For example, tert-butylethyl ether is prepared by treating ethyl bromide with sodium tert-butoxide.
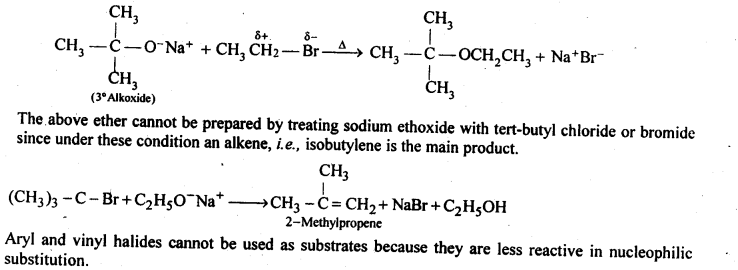
11.26. How is 1-propoxypropane synthesised from propane-1-ol? Write mechanism of the reaction. (C.B.S.E. Sarnie Paper 2015)
Ans: Two methods can be used for the synthesis of 1-propoxypropane from propan-1-ol
By Williamson’s synthesis
The halogen derivative such as bromoderivative and sodium salt of the alcohol take part in the Williamson’s synthesis
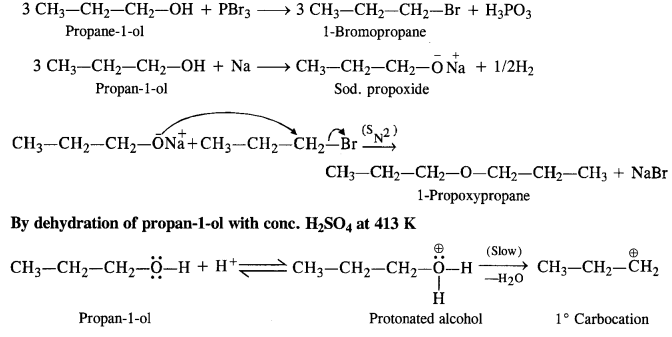
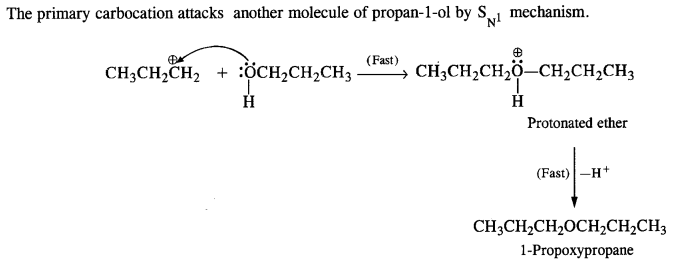
11.27. Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Ans: Acid catalysed dehydration of primary alcohols to ethers occurs by SN2 reaction involving nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.
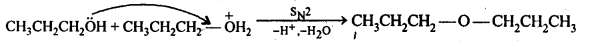
Under these conditions, 2° and 3° alcohols, however, give alkenes rather than ethers. The reason being that due to steric hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not occur. Instead protonated 2° and 3° alcohols lose a molecule of water to form stable 2° and 3° carbocation. These carbocations prefer to lose a proton to form alkenes rather than undergoing nucleophilic attack by alcohol molecules to form ethers.
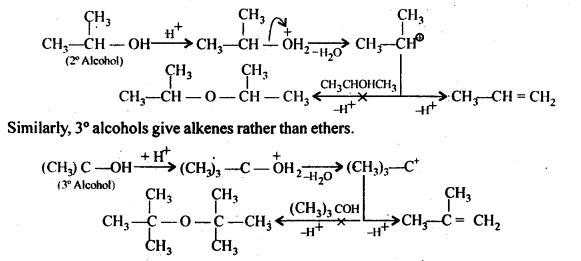
11.28. Write the equation of the reaction of hydrogen iodide with (i)1-propoxypropane (ii)methoxybenzene, and (iii)benzyl ethyl ether
Ans:
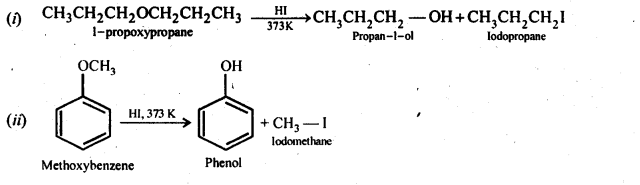
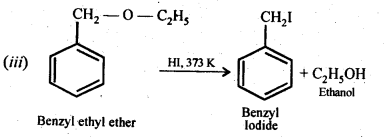
11.29. Explain the fact that in alkyl aryl ethers, alkoxy group :
(i) activates the benzene ring towards electrophilic substitution.
(ii) directs the incoming substituents towards ortho and para positions in the ring.
Ans:
(i) The alkoxy group (RO -) with lone electron pairs on the oxygen atom activates the ortho and para positions in the ring by + M (or + R) effect as shown below :
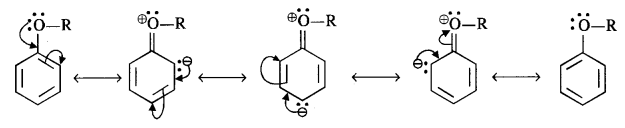
As the ortho and para positions in the ring become points of high electron density, the electrophiles prefer to attack these positions.
(ii) The alkoxy group directs the incoming group which is an electrophile towards the ortho and para positions in the ring. As a result, a mixture of isomeric products is formed.
11.30. Write the mechanism of the reaction of HI with methoxymethane.
Ans: When equimolar amounts of HI and methoxy methane are reacted, a mixture of methyl alcohol and methyl iodide is formed by the following mechanism:
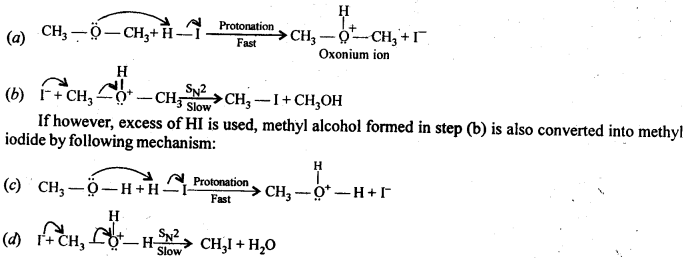
11.31. Write equations of the following reactions:
(i) Friedel-Crafts reaction -alkylation of anisole
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium
(iv) Friedel-Craft’s acetylation of anisole.
Ans:
11.32. Show how will you synthesise the following from appropriate alkenes.
Ans: All the alcohols are formed by the hydration of alkenes in the acidic medium. The addition follows Markownikov’s rule. 1-Methylcyclohexene can be used in the reaction.
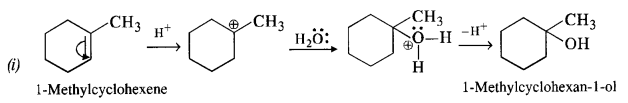
(ii) 4-Methylpent-3-ene upon hydration in the acidic medium will give the desired alcohol.
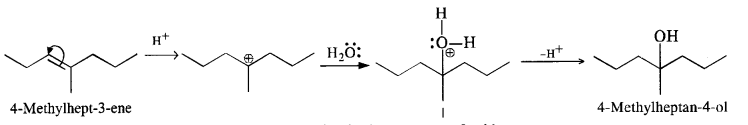
(iii) Pent-2-ene gives the desired alcohol upon hydration in the presence of acid.
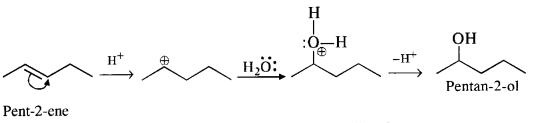
(iv) The cyclic alkene used in this reaction is 2-cyclohexylbut-2-ene.
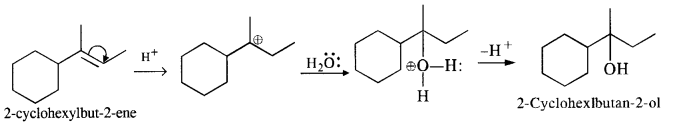
11.33. When 3-methylbutant 2-ol is treated with HBr, the following reaction takes place:
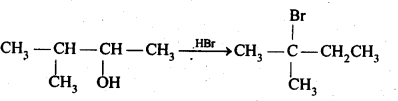
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
Ans:
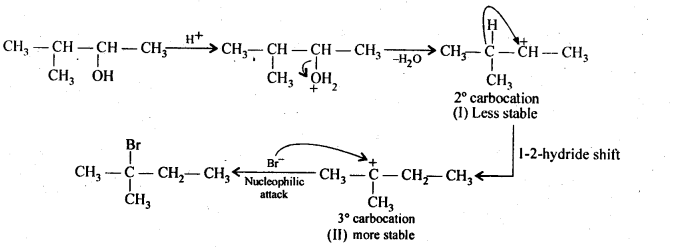
Protonation of the given alcohol followed by loss of water gives a 2° carbocation(I), which being unstable rearranges by 1,2-hydride shift to form the more stable 3° carbocation (II). Nucleophilic attack by Br ion on this carbocation (II) gives the final product.
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols Phenols and Ether:
| Section Name | Topic Name |
| 11 | Alcohols, Phenols and Ethers |
| 11.1 | Classification |
| 11.2 | Nomenclature |
| 11.3 | Structures of Functional Groups |
| 11.4 | Alcohols and Phenols |
| 11.5 | Some Commercially Important Alcohols |
| 11.6 | Ethers |
NCERT SolutionsCBSE Sample Papers ChemistryClass 12 Chemistry
NCERT TEXTBOOK QUESTIONS SOLVED
11.1. Classify the following as primary, secondary and tertiary alcohols.


Ans: Primary alcohols: (i), (ii), (iii)
Secondary alcohols: (iv), (v)
Tertiary alcohols: (vi)
11.2. Identify aliylic alcohols in the above examples.
Ans: (ii) and (iv) i.e. H2C=CH – CH2OH and
11.3. Name the following compounds according to IUPAC system.
Ans: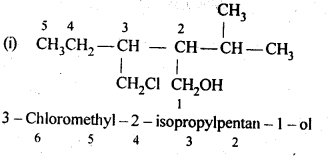

11.4. Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal ?
Ans:

11.5. Write structures of the products of the following reactions:
Ans:
11.6. Give structures of the products you would expect when each of the following alcohol reacts with (a)HCl-ZnCl2 (b)HBrand (c) SOCl2
(i)Butan-1-ol
(ii)2-Methylbutan-2-ol
Ans:
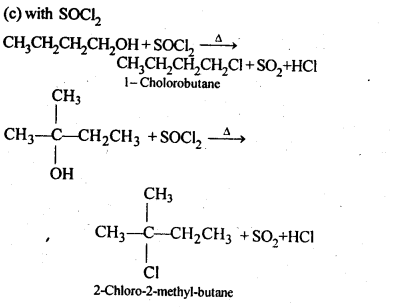
11.7. Predict the major product of acid catalysed dehydration of
(i) 1-nicthylcyclohcxanoland
(ii) butan-1-ol
Ans:
11.8. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Ans:
The resonance structures of o-and p- nitrophenoxide ions and phenoxide ion are given below:

11.9; Write the equations involved in the following reactions:
(i) Reimer-Tiemann reaction
(ii) Kolbe’s reaction
Ans: (i) Reimer-Tiemann reaction
11.10. Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Ans: In Williamsons’s synthesis, the alkyl halide should be primary. Thus, the alkyl halide should be derived from ethanol and the alkoxide ion from 3-methylpentan-2-ol. The synthesis is as follows
11.11. Which of the following is an appropriate set of reactants for the preparation of l-methoxy-4- nitrobenzene and why?
Ans:
11.12. Predict the products of the following reactions:
Ans:
NCERT EXERCISES
11.1. Write IUPAC names of the following compounds: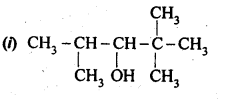


Ans: (i) 2,2,4-Trimethylpentan-3-ol
(ii) 5-Ethylheptane-2,4-dioI
(iii) Butane-2,3-diol
(iv) Propane-1,2,3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2,5-DimethylphenoI
(viii) 2,6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane
11.2. Write structures of the compounds whose IUPAC names are as follows:
(i)2-Methylbutan-2-ol
(ii)l-Phcnylpropan-2-ol
(iii)3,5-DimethyIhexane-l,3,5-triol
(iv)2,3-Dicthylphenol
(v)1-Ethoxypropane
(vi)2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpcntan-3-ol
(ix)Cyclopcnt-3-en-l-ol
(x)4-ChIoro-3-ethylbutan-l-ol
Ans:

11. 3. (a) Draw the structural formulas and write IUPAC names of all the isomeric alkanols with the molecular formula CsH12O
(b) Classify the isomers of alcohols given in part (a) as primary, secondary and tertiary alcohols.
Ans:
(a) The molecular formula C5H120 represents eight isomeric alkanols. These are :
(b) Primary: (i), (ii), (iii), (iv) ; Secondary :(v), (vi), (viii) ; Tertiary : (vii)
11.4. Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Ans: The molecules of butane are held together by weak van der Waal’s forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.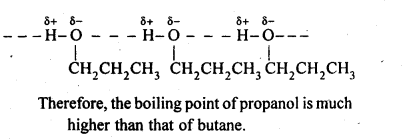
11.5. Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Ans: Alcohols can form hydrogen bonds with water and by breaking the hydrogen bonds already existing between water molecules. Therefore, they are soluble in water.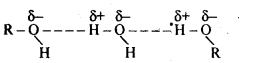
On die other hand, hydrocarbons cannot from hydrogen bonds with water and hence are insoluble in water.
11.6. What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Ans: The addition of diborane to alkenes to form trialkyl boranes followed by their oxidation with alkaline hydrogen peroxide to form alcohols is called hydroboration-oxidation. For example,
11.7. Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Ans: The three isomers are:
11.8. While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Ans: 0-N itrophenol is steam volatile due to chelation (intramolecular H – bonding) and hence can be separated by steam distillation from/Miitrophenol which is hot steam volatile because of intermolecular H-bonding.
11.9. Give the equations of the reaction for the preparation of phenol from cumene.
Ans: This process has a great industrial importance because it gives the preparation of two very useful compounds i.e. phenol and acetone. The raw materials are benzene and propene and it initially proceeds by Friedel Crafts alkylation of benzene.
Oxygen is bubbled through the above solution to form cumene hydroperoxide which is decomposed with aqueous acid
solution to form phenol and acetone as follows:
11.10. Write chemical reaction for the preparation of phenol from chlorobenzene.
Ans:
11.11. Write the mechanism of hydration of ethene to yield ethanol.
Ans: Direct addition of H20 to ethene in presence of an acid does not occur. Indirectly, ethene is first passed through concentrated H2S04, when ethyl hydrogen sulphate is formed.
11.12. You are given benzene, cone. H2S04and NaOH. Write the equations for the preparation of phenol using these reagents.
Ans:
11.13. Show how will you synthesise
(i) 1 -phenylethanol from a suitable alkene.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) Pentan-l-ol using a suitable alkyl halide?
Ans:
11.14. Give two reactions that show the acidic nature of phenol. Compare its acidity with that of ethanol.
Ans: The reactions showing acidic nature of phenol are:
(a) Reaction with sodium: Phenol reacts with active metals like sodium to liberate H, gas.
(b) Reaction with NaOH: Phenol dissolves in NaOH to form sodium phenoxide and water.
Phenol is more acidic than ethanol. This is due to the reason that phenoxide ion left after the loss of a proton from phenol is stabilized by resonance, while ethoxide ion left after less of a proton from ethanol, is not.
11.15. Explain why is orthonitrophenol more acidic than orthomethoxyphenol?
Ans: Nitro (NO2) group is an electron withdrawing group while methoxy (OCH3) group is electron releasing in nature. The release of H+ ion is therefore, easier from o-nitrophenol while it is quite difficult from o-methoxyphenol. Apart form that, o-nitrophenoxide ion is stabilised due to resonance o-nitrophenol is steam volatile while p-nitrophenol is not. This is on account of intramolecular hydrogen bonding in the molecules of o-nitrophenol. As a result, its boiling point is less than that of p-nitrophenol in which the molecules are linked by intermolecular hydrogen bonding.
It is interesting to note that in the substituted phenols, the nature and position of the substituent influences the boiling point of phenol.
For example: .o-nitrophenol is steam volatile while p-nitrophenol is not. This is supported by the fact that the boiling point temperature of o-nitrophenol (100°C) is less than that of p-nitrophenol, (279°C). In o-nitrophenol, there is intramolecular hydrogen bonding in OH and NO2 groups placed in a adjacent positions. However, these are linked by intermolecular hydrogen bonding in the p-isomers. It is quite obvious that extra energy is needed to the cleave the hydrogen bonds in the p-isomer. Consequently, its boiling point is more.
o-nnrophenol with lower boiling point is steam volatile while p-nitrophenol is not. This helps in the separation of the two isomers present in the liquid mixture. On passing steam, o-nitropbenol volatilises and its vapours rise alongwith steam and after condensation, collect in the receiver p-nitrophenol is left behind in the distillation flask. e-nkrophenol p-nnrophenol.
On the contrary, o-methoxyphenoxide is destabilised since the electron density on the negatively charged oxygen tends to increase due to the electron releasing tendency of the methoxy(OCH3) group.
In the light of the above discussion, we may conclude that o-nitrophenol is a stronger acid (pKa = 7-23) than o-methoxyphenl (pKa = 9.98)
11.16. Explain how does the – OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans: Phenol may be regarded as a resonance hybrid of structures I-V, shown below.
As a result of +R effect of the -OH group, the electron density in the benzene ring increases thereby facilitating the attack of an electrophile. In other words, presence of -OH group, activates the benzene ring towards electrophilic substitution reactions. Further, since the electron density is relatively higher at the two o-and one p-position, therefore electrophilic substitution occurs mainly at o-and p-positions.
11.17. Give equations of the following reactions:
(i) Oxidation of propan-l-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 acid with phenol
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Ans:
11.18. Explain the following with an example
(i) Kolbe’s reaction
(ii) Reimer – Tiemann reaction –
(iii) Williamson ether synthesis
(iv) Unsymmetrical ether
Ans: (i) Kolbe’s reaction: Sodium phenoxide when heated with C02 at 400K under a pressure of 4-7 atmospheres followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the major product along with a small amount of 4-hydroxybenzoic acid.This reaction is called Kolbe’s reaction.
(ii) Reimer-Tiemann reaction: Treatment of phenol with CHC13 in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicyialdehyde) as the major product. This reaction is called Reimer-Tiemann reaction.
(iii) Williamson’s ether synthesis: It involves the treatment of an alkyl halide with a suitable sodium alkoxide to obtain ethers. The sodium alkoxide needed for the purpose is prepared by the action of sodium on a suitable alcohol. In this reaction alkyl halide should primary. Secondary and tertiary halides will predominantly give an alkene.![]()
(iv) Unsymmetrical ether: If the alkyl or aryl groups attached to the oxygen atom are different, ethers are called unsymmetrical ethers. For example, ethyl methyl ether, methyl phenyl ether, 4-chlorophenyl- 4-nitrophenyl ether, etc.
11.19. Write the mechanism of acid dehydration of ethanol to yield ethene.
Ans: The mechanism of dehydration of alcohols to form alkenes occur by the following three steps:
11.20. How are the following conversions carried out?
(i) Propane → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl mag. chloride → Propan-1-ol
(iv) Methyl mag. bromide → 2-Methylpropan-2-ol.
Ans:
11.21. Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Brominationofphenolto2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-oI to propene.
(vi) Butan-2-one to butan-2-oL .
Ans: (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
11.22. Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Ans: Ethanol undergoes intermolecular H-bonding due to the presence of a hydrogen atom attached to the electronegative oxygen atom. As a result, ethanol exists as associated molecules.
Consequently, a large amount of energy is required to break these hydrogen bonds. Therefore, the boiling point of ethanol is higher than that of methoxymethane which does not form H-bonds.
11.23. Give IUPAC names of the following ethers.
Ans: (i)1-Ethoxy-2-methylpropane
(ii) 2-Chlorlo-l-methoxy ethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 1 -Ethoxy-4 -4 – dimethyl cyclohexane
(vi)Ethoxybenzene
11,24. Write the names of the reagents and equations for the preparation of the following ethers by Williamson’s synthesis :
(i) 1-Propoxypropane
(ii) 2-Methoxy-2-methylpropane
(iii) Ethoxybenzene
(iv) Methoxyethane.
Ans:
11.25. Illustrate with examples the limitations of Willamson synthesis for the preparation of certain types of ethers.
Ans: Williamson’s synthesis is a versatile method for the synthesis of both symmetrical and unsymmetrical ethers. However, for the synthesis of unsymmetrical ethers, a proper choice of reactants is necessary. Since Williamson’s synthesis occurs by SN2 mechanism and primary alkyl halides are most reactive in Sn2 reaction, therefore, best yields of unsymmetrical ethers are obtained when the alkyl halides are primary and the alkoxide may be primary, secondary or tertiary. For example, tert-butylethyl ether is prepared by treating ethyl bromide with sodium tert-butoxide.
11.26. How is 1-propoxypropane synthesised from propane-1-ol? Write mechanism of the reaction. (C.B.S.E. Sarnie Paper 2015)
Ans: Two methods can be used for the synthesis of 1-propoxypropane from propan-1-ol
By Williamson’s synthesis
The halogen derivative such as bromoderivative and sodium salt of the alcohol take part in the Williamson’s synthesis

11.27. Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Ans: Acid catalysed dehydration of primary alcohols to ethers occurs by SN2 reaction involving nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.![]()
Under these conditions, 2° and 3° alcohols, however, give alkenes rather than ethers. The reason being that due to steric hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not occur. Instead protonated 2° and 3° alcohols lose a molecule of water to form stable 2° and 3° carbocation. These carbocations prefer to lose a proton to form alkenes rather than undergoing nucleophilic attack by alcohol molecules to form ethers.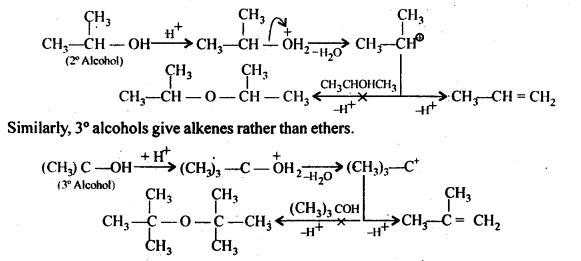
11.28. Write the equation of the reaction of hydrogen iodide with (i)1-propoxypropane (ii)methoxybenzene, and (iii)benzyl ethyl ether
Ans:

11.29. Explain the fact that in alkyl aryl ethers, alkoxy group :
(i) activates the benzene ring towards electrophilic substitution.
(ii) directs the incoming substituents towards ortho and para positions in the ring.
Ans:
(i) The alkoxy group (RO -) with lone electron pairs on the oxygen atom activates the ortho and para positions in the ring by + M (or + R) effect as shown below :
As the ortho and para positions in the ring become points of high electron density, the electrophiles prefer to attack these positions.
(ii) The alkoxy group directs the incoming group which is an electrophile towards the ortho and para positions in the ring. As a result, a mixture of isomeric products is formed.
11.30. Write the mechanism of the reaction of HI with methoxymethane.
Ans: When equimolar amounts of HI and methoxy methane are reacted, a mixture of methyl alcohol and methyl iodide is formed by the following mechanism:
11.31. Write equations of the following reactions:
(i) Friedel-Crafts reaction -alkylation of anisole
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium
(iv) Friedel-Craft’s acetylation of anisole.
Ans:

11.32. Show how will you synthesise the following from appropriate alkenes.
Ans: All the alcohols are formed by the hydration of alkenes in the acidic medium. The addition follows Markownikov’s rule. 1-Methylcyclohexene can be used in the reaction.
(ii) 4-Methylpent-3-ene upon hydration in the acidic medium will give the desired alcohol.
(iii) Pent-2-ene gives the desired alcohol upon hydration in the presence of acid.
(iv) The cyclic alkene used in this reaction is 2-cyclohexylbut-2-ene.
11.33. When 3-methylbutant 2-ol is treated with HBr, the following reaction takes place: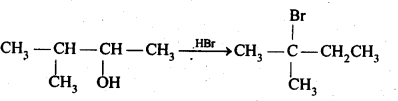
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
Ans:
Protonation of the given alcohol followed by loss of water gives a 2° carbocation(I), which being unstable rearranges by 1,2-hydride shift to form the more stable 3° carbocation (II). Nucleophilic attack by Br ion on this carbocation (II) gives the final product.
Class 12 Chemistry Chapter 14 Biomolecules
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules:
Section Name
Topic Name
14
Biomolecules
14.1
Carbohydrates
14.2
Proteins
14.3
Enzymes
14.4
Vitamins
14.5
Nucleic Acids
14.6
Hormones
NCERT INTEXT QUESTIONS
14.1. Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain.
Ans: The .solubility of a solute in a given solvent follows the rule ‘ Like dissolves like’.Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water. As a result of this extensive intermoleeular H-bonding, glucose and sucrose are soluble in water.On the other hand, benzene and cyclohexane do not contain -OH bonds and hence do not form H-bonds with water. Moreover, they are non-polar molecules and hence do not dissolve in polar water molecules.
14.2. What are the expected products of hydrolysis of lactose?
Ans: Lactose being a disaccharide gives two molecules of monosaccharides Le. one molecule each of D-(+) – glucose and D-(+)-galactbse.
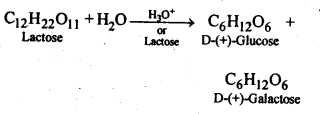
14.3. How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Ans: The cyclic hemiacetal form of glucose contains an -OH group at C-l which gets hydrolysed in aqueous solution to produce open chain aldehydic form which then reacts with NH2OH -to form corresponding oxime. Thus, glucose contains an aldehydic group. However, when glucose is reacted with acetic anhydride, the -OH group at C-l along with the other -OH groups at C-2, C-3, C-4 and C-6 form a pentaacetate.
Since the penta acetate of1 glucose does not contain a free -OH group at C-l, it cannot get hydrolysed in aqueous solution to produce open chain aldehydic form and hence glucose pentaacetate does not react with NH2OH to form glucose oxime. The reactions are shown as:
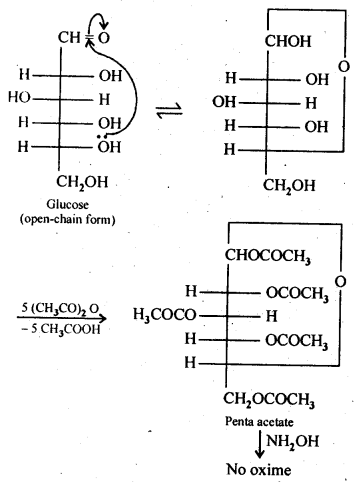
14.4. The melting points and solubility in water of a-amino acids are generally higher than those of corresponding haloacids. Explain.
Ans: a-amino acids as we all know, are dipolar in nature (N+ H3-CHR-COO– ) and have strong dipolar interactions. As a result, these are high melting solids. These are also involved in intermolecular hydrogen bonding with the molecules of water and are therefore, water soluble. On the contrary, the haloacids RCH(X)COOH are not dipolar like a-amino acids. Moreover, only the carboxyl group of haloacids are involved in hydrogen bonding with the molecules of water and not the halogen atoms. These have therefore, comparatively less melting points and are also soluble in water to smaller extent.
14.5. Where does the water present in the egg go after boiling the egg?
Ans: When egg is boiled, proteins first undergo denaturation and then coagulation and the water present in the egg gets absorbed in coagulated protein, probably through H- bonding
14.6. Why cannot Vitamin C be stored in our body?
Ans: Vitamin C cannot be stored in the body because it is water soluble and is, therefore, easily excreted in urine.
14.7. Which products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Ans: Upon hydrolysis, nucleotide from DNA would form 2-deoxyribose and phosphoric acid along-with thymine.
14.8. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Ans: A DNA molecule has two strands in which the four complementary bases pair each other, i.e., cytosine (C) always pair with guanine (G) while thymine (T) always pairs with adenine (A). Thus, when a DNA molecule is hydrolysed, the molar amounts of cytosine is always equal to that of guanine and that of adenine is always equal to thymine.In RNA, there is no relationship between the quantities of four bases (C, G, A and U) obtained, therefore, the base pairing principle, i.e. A pairs with U and C pairs with G is not followed. Therefore, unlike DNA, RNA has a single strand.
NCERT EXERCISES
14.1. What are monosaccharides ?
Ans: Monosaccharides are carbohydrates Which cannot be hydrolysed to smaller molecules.Their general formula is (CH2O)n Where n=3-7 These are of two types: Those which contain an aldehyde group (-CHO) are called aldoses and those which contain a keto (C=O) group are called ketoses.
They are further classified as trioses , tetroses ,pentoses , hexoses and heptoses according as they contain 3,4,5,6, and 7 carbon atoms respectively.For example.
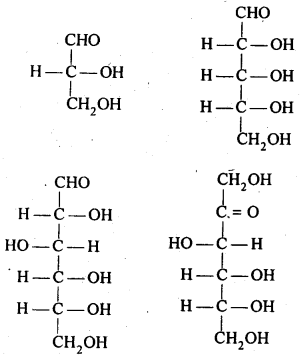
14.2. What are reducing sugars?
Ans: Reducing sugars are those which can act as reducing agents. They contain in them a reducing group which may be aldehydic (-CHO) or ketonic (>C=0) group. The characteristic reactions of reducing sugars are with Tollen’s reagent and Fehling solution. Non-reducing sugars donot give these reactions. For example, glucose, fructose, lactose etc. are reducing sugars. Sucrose is regarded as a non-reducing sugar because both glucose and fructose are linked through their aldehydic and ketonic groups by glycosidic linkage. Since these groups are not free, sucrose is a non-reducing sugar.
14.3. Write two main functions of carbohydrates in plants.
Ans: Two major functions of carbohydrates in plants are following
(a)Structural material for plant cell walls: The polysaccharide cellulose acts as the chief structural material of the plant cell walls.
(b)Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and act as the reserve food material for the tiny plant till it is capable of making its own food by photosynthesis.
14.4. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans: Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose. Disaccharides: Maltose and lactose.
14.5. What do you understand by the term glycosidic linkage?
Ans: The ethereal or oxide linkage through which two monosaccharide units are joined together by the loss of a water molecule to form a molecule of disaccharide is called the glycosidic linkage. The glycosidic linkage in maltpse molecule is shown below:
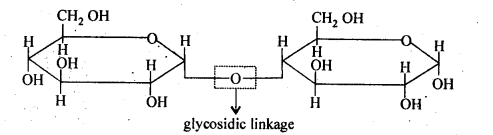
14.6. What is glycogen? How is it different from starch?
Ans: The carbohydrates are stored in animal body as glycogen. It is also called animal starch and its structure is similar toamylopectin which means that it is a branched chain polymer of α-D-glucose units in which the chain is formed by C1 – C4 glycosidic linkage whereas branching occurs by the formation of C1– C6 glycosidic linkage. One main difference between glycogen and amylopectin is the length of the chain. In amylopectin, the chain consists of 20 – 25 α – D – glucose molecules whereas in glycogen, there are 10 -14 molecules of α – D – glucose present. Glycogen is more branched than amylopectin. It is present mainly in liver, muscles and also in brain. Glycogen gets converted into glucose when the body needs it with the help of certain enzymes present in the body. Glycogen has also been found to be present in yeast and fungi.
Starch is a major source of carbohydrates which are very much essential to the human body since they supply energy to the body. It occurs as granules mainly in seeds, fruits, tubers and also in the roots of the plants. The chief commercial sources of starch are wheat, maize, rice, potatoes etc.
14.7. What are the hydrolysis products of (i) sucrose, and (ii) lactose?
Ans: Both sucrose and lactose are disaccharides. Sucrose on hydrolysis gives one molecule each of glucose and fructose but lactose on hydrolysis gives one molecule each of glucose and galactose.
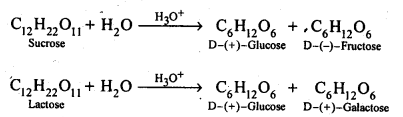
14.8. What is the basic structural difference between starch and cellulose?
Ans: Starch consists of amylose and amylopectin. Amylose is a linear polymer of α-D-glucose while cellulose is a linear polymer of β -D- glucose. In amylose, C -1 of one glucose unit is connected to C – 4 of the other through α-glycosidic linkage. However in cellulose, C – 1 of one glucose unit is connected to C-4 of the other through β – glycosidic linkage. Amylopectin on the other hand has highly branched structure.
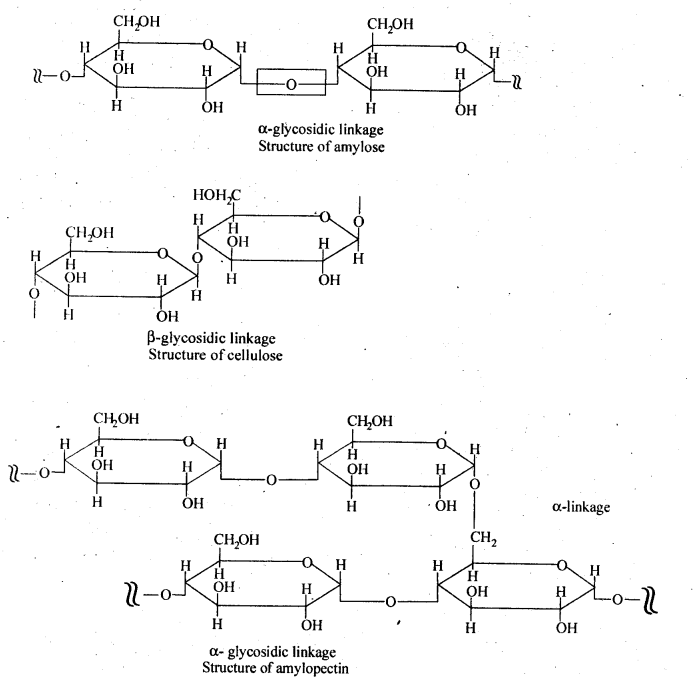
14.9. What happens when D-glucose is treated with . the following reagents.
(i) HI
(ii) Bromine water
(iii) HNO3
Ans:
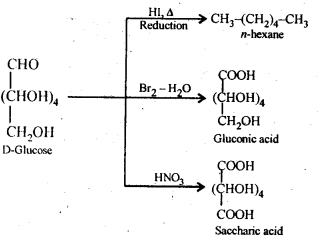
14.10. Enumerate the reactions of D-glucose which cannot be explained with open chain structure. (C.B.S.E. Delhi 2008, C.B.S.E. Sample Paper 2011)
Ans:
Open chain structure of D-glucose contains a free aldehydic group (- CHO). However, it does not give the following reactions:
- D(+) glucose does not react with 2, 4 D.N.P.
- D(+) glucose does not react with NaHSO3.
- D(+) glucose does not restore the pink colour to Schiff’s reagent.
- Penia acetyl glucose formed by carrying acetylation with acetic anhydride does not react with hydroxyl amine
(NH2OH) which is the characteristic reaction of all aldehydes.
- D( +) glucose is found to exist in two different crystalline forms which are named as α and β. Both these forms have actually been isolated. For example, α form with m.p. 419 K is obtained by the crystallisation of the saturated solution of glucose prepared at 303 K. Similarly, β-form with m.p. 423 K is isolated by carrying out the crystallisation of the saturated solution of glucose prepared at 371 K. Apart from that the a-form has a specific rotation (α) equal to + 112° while the β- form has specific rotation (α) equal to + 19°.
In the light of the limitations stated above, Tollen stated that an open chain structure for D(+) glucose is probably not practicable. He proposed a cyclic structure which is a hemiacetal structure. In this structure, the aldehydic (CHO) group
is involved in the form of a ring with the -OH group attached to C5 carbon. It is a six membered ring, often called ô-
oxide ring. The ring structure accounts for the two isomeric forms a and shown below.
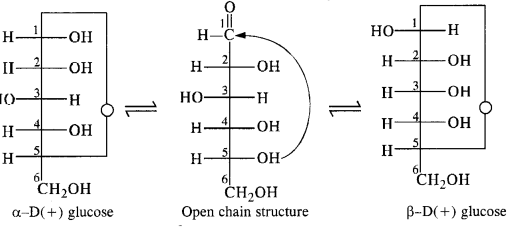
14.11. What are essential and non-essential amino acids? Give two examples of each type.
Ans: α-Amino acids which are needed for good health and proper growth of human beings but are not synthesized by the human body are called- essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, α-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid etc.
14.12. Define the following as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
Ans: (i) Peptide bond: Proteins are condensation polymers of α-amino acids in which the same or different α-amino acids are joined by peptide bonds. Chemically, a peptide bond is an amide linkage formed between – COOH group of one α-amino acid and -NH-, group of the other α-amino acid by lo;ss of a molecule of water. For example,
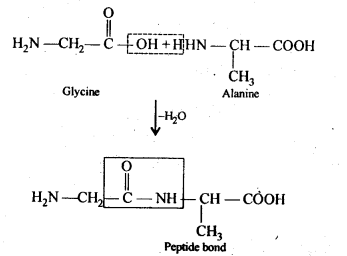
(ii) Primary structure: Proteins may contain one or more polypeptide chains. Each . polypeptide chain has a large number of α-amino acids which are linked to one another in a specific manner. The specific sequence in which the various amino acids present in a protein linked to one another is called its primary structure. Any change in the sequence of α-amino acids creates a different protein.
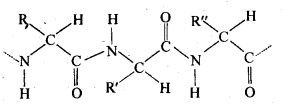
(iii) Denaturation: Each protein in the biological system has a unique three-dimensional structure and has specific biologicalactivity. This is called native form of a protein. When a protein in its native form is subjected to a physical change such as change in temperature or a chemical change like change in pH, etc., hydrogen bonds gets broken. As a result, soluble forms of proteins such as globular proteins undergo coagulation or precipitation to give fibrous proteins which are insoluble in water. This coagulation also results in loss of biological activity of the proteins and this loss in biological activity, is called denaturation. During denaturation, 2° and 3° structures of proteins are destroyed but 1° structure remains intact.
The most common example of denaturation of proteins is the coagulation of albumin present in the white of an egg. When the egg is boiled hard, the soluble globular protein present in it is denatured and is converted into insoluble fibrous protein.
14.13. What are the common types of secondary structure of proteins?
Ans:
Secondary structure of protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
- α-helix structure
- β-pleated sheet structure.
Secondary Structure of Proteins:
The long, flexible peptide chains of proteins are folded into the relatively rigid regular conformations called the
secondary structure. It refers to the conformation which the polvpeptide chains assume as a result of hydrogen bonding
between the > C= O and > N-H groups of different peptide bonds.
The type of secondary structure a protein will acquire, in general depends upon the size of the R-group. If the size of the
R-groups are quite large, the protein will acquire ct-helix structure. If on the other hand, the size of the R-groups are relatively
smaller, the protein will acquire a β – flat sheet structure.
(a) α-Helix structure: If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group
of one amino acid unit and the > N-H group of the fourth amino acid unit within the same chain. As such the polypeptide
chain coils up into a spiral structure called right handed ct- helix structure. This type of structure is adopted by most of the
fibrous structural proteins such as those present in wool, hair and muscles. These proteins are elastic i.e., they can be
stretched. During this process, the weak hydrogen bonds causing the a – helix are broken. This tends to increase the length of
the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.
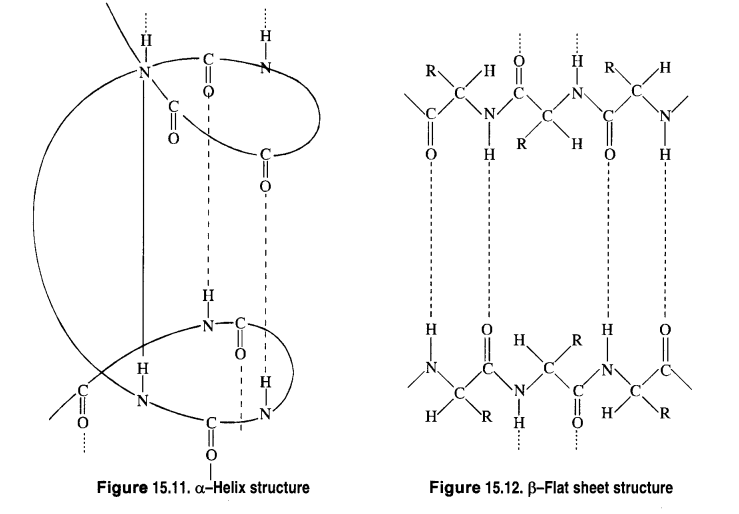
(b) β—Flat sheet or β—Pleated sheet structure: If R-groups are relatively small, the peptide chains lie side by side in a zig
zag manner with alternate R-groups on the same side situated at fixed distances apart. The two such neighbouring chains are held together by intermolecular hydrogen bonds. A number of such chains can be inter-bonded and this results in the formation of a flat sheet structure These chains may contract or bend a little in order to accommodate moderate sized R-groups. As a result, the sheet bends into parallel folds to form pleated sheet structure known as β – pleated sheet structure. These sheets are then stacked one above the other like the pages of a book to form a three dimensional structure. The protein fibrion in silk fibre has a β – pleated sheet structure. The characteristic mechanical properties of silk can easily be explained on the basis of its β – sheet structure. For example, silk is non-elastic since stretching leads to pulling the peptide covalent bonds. On the other hand, it can be bent easily like a stack of pages because during this process, the sheets slide over each other.
14.14. What types of bonding helps in stabilising the α-helix structure of proteins?
Ans: α-helix structure of proteins is stabilised through hydrogen bonding. (a) α -Helix structure. If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group of one amino acid unit and the > N- H group of the fourth amino acid unit within the same chain. As such the polypeptide chain coils up into a spiral structure called right handed a—helix structure. This type of structure is adopted by most of the fibrous structural proteins such as those present in wool, hair and muscles. These proteins are elastic i.e., they can be stretched. During this process, the weak hydrogen bonds causing the α-helix are broken. This tends to increase the length of the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.
14.15: Differentiate between globular and fibrous proteins.
Ans. (i) Fibrous proteins: These proteins consist of linear thread like molecules which tend to lie side by side (parallel) to form fibres. The polypeptide chains in them are held together usually at many points by hydrogen bonds and some disulphide bonds. As a result,intermolecular forces of attraction are very’ strong and hence fibrous proteins are insoluble in water. Further, these proteins are stable to moderate changes in temperature and pH. Fibrous proteins serve as the chief structural material of animal tissues.For example, keratin in skin, hair, nails and wool, collagen in tendons, fibrosis in silk and myosin in muscles.
(ii) Globular proteins: The polypeptide chain in these proteins is folded around itself in such a way so as to give the entire protein molecule an almost spheroidal shape. The folding takes place in such a manner that hydrophobic (non-polar) parts are pushed inwards and hydrophilic (polar) parts are pushed outwards. As a result, water molecules interact strongly with the polar groups and hence globular protein are water soluble. As compared to fibrous proteins, these are very sensitive to small changes of temperature and pH. This class of proteins include all enzymes, many hormones such as insulin from pancreas, thyroglobulin from thyroid gland, etc.
14.16. How do you explain the amphoteric behaviour of amino acids?
Ans: Amino acids contain an acidic (carboxyl group) and basic (amino group) group in the same molecule. In aqueous solution, they neutralize each other. The carboxyl group loses a proton while the amino group accepts it. As a result, a dipolar or zwitter ion is formed.
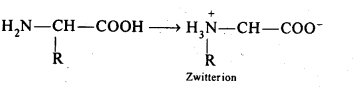
In zwitter ionjc form, a-amino acid show amphoteric behaviour as they react with both acids and bases.
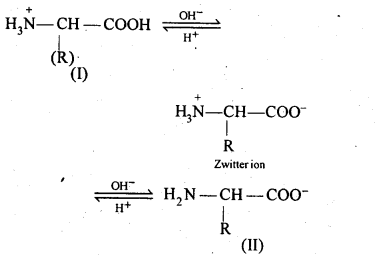
14.17. What are enzymes?
Ans: Enzymes are biological catalyst. Each biological reaction requires a different enzyme. Thus, as compared to conventional catalyst enzymes are very specific and efficient in their action. Each type of enzyme has its own specific optimum conditions of concentration, pH and temperature at which it works best.
14.18. What is the effect of denaturation on the structure of proteins?
Ans: Denaturation of proteins is done either by change in temperature (upon heating) or by bringing a change in the pH of the medium. As a result, the hydrogen bonding is disturbed and the proteins lose their biological activity i.e., their nature changes. During the denaturation, both the tertiary and secondary structures of proteins are destroyed while the primary structures remain intact.
14.19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans: Vitamins are classified into two groups depending upon their solubility in water or fat: (i) Water soluble vitamins: These include vitamin B-complex (B1, B2, B5, i.e., nicotinic acid,B6, B12, pantothenic acid, biotin, i.e., vitamin H and folic acid) and vitamin C.
(ii) Fat soluble vitamins: These include vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues. Vitamin K is responsible for coagulation of blood.
14.20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Ans: Vitamin A is essential for us because its deficiency causes xerophthalmia (hardening of cornea of eye) and night blindness.
Sources: Fish liver oil, carrots, butter, milk, etc. Vitamin C is essential for us because its deficiency causes scurvy (bleeding of gums) and pyorrhea (loosening and bleeding of teeth). Sources: Citrous fruits, amla, green leafy vegetables etc.
14.21. What are nucleic acids ? Mention their two important functions.
Ans: Nucleic acids are biomolecules which are found in the nuclei of all living cell in form of nucleoproteins or chromosomes (proteins contains nucleic acids as the prosthetic group).
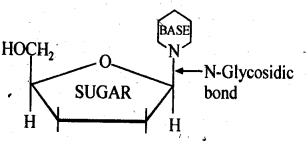
Nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid.(RNA).
The two main functions of nucleic acids are:
(a) DNA is responsible for transmission of hereditary effects from one generation to another. This is due to its unique property of replication, during cell division and two identical DNA strands are transferred to the daughter cells.
(b) DNA and RNA are responsible for synthesis of all proteins needed for the growth and maintenance of our body. Actually the proteins are synthesized by various RNA molecules (r-RNA, m-RNA) and t-RNA) in the cell but the message for the synthesis of a particular protein is coded in DNA.
14.22. What is the difference between a nucleoside and a nucleotide?
Ans: A nucleoside contains only two basic components of nucleic acids i.e., a pentose sugar and a nitrogenous base. It is formed when 1- position of pyrimidine (cytosine, thiamine or uracil) or 9-position of purine (guanine or adenine) base is attached to C -1 of sugar (ribose or deoxyribose) by a β-linkage. Nucleic acids are also called polynucleotides since the repeating structural unit of nucleic acids is a nucleotide.
A nucleotide contains all the three basic . components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and a nitrogenous base. These are obtained by esterification of C5, – OH group of the pentose sugar by phosphoric acid.
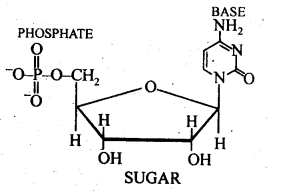
14.23. The two strands in DNA are not identical but are complementary. Explain.
Ans: The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (cytosine) through three H-bonds, (i.e.,C = G) and between A (adenine) and T (thiamine) through two H-bonds (i.e., A = T). Due to this base -pairing principle, the sequence of bases in one strand automatically fixes the sequence of bases in the other strand. Thus, the two strands are complimentary and not identical.
14.24. Write the important structural and functional differences between DNA and RNA.
Ans:
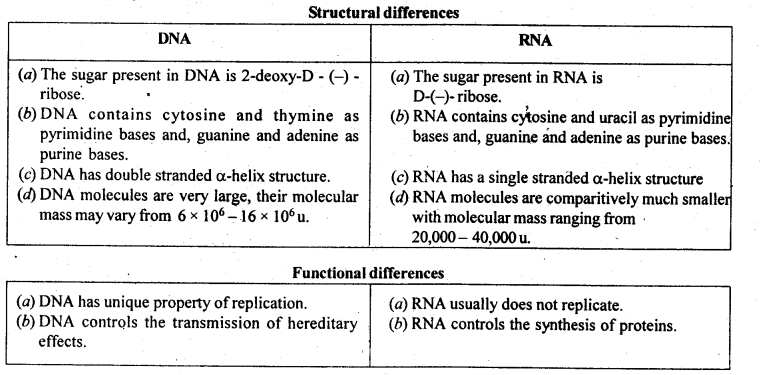
14.25. What are the different types of RNA found in the cell?
Ans: There are three types of RNA:
(a) Ribosomal RNA (r RNA)
(b) Messenger RNA (m RNA)
(c) Transfer RNA (t RNA)
Class 12 Chemistry Chapter 15 Polymers:
Section Name
Topic Name
15
Polymers
15.1
Classification of Polymers
15.2
Types of Polymerisation Reactions
15.3
Molecular Mass of Polymers
15.4
Biodegradable Polymers
15.5
Polymers of Commercial Importance
NCERT INTEXT QUESTIONS
15.1. What are polymers?
Ans: Polymers are high molecular mass substances (103 — 107u) consisting of a very large number of simple repeating structural units joined together through covalent bonds in a linear fashion. They are also called macromolecules. Ex: polythene, nylon 6,6, bakelite, rubber, etc.
15.2. How are polymers classified on the basis of structure?
Ans: On the basis of structure, polymers are classified into three types. These are linear chain polymers, branched chain polymers and crossedlinkedpolymers.
1. Linear chain polymers: In this case, the monomer units are linked to one another to form long linear chains. These linear chains are placed one above the other and are closely packed in space. The close packing results in high densities, tensile strength and also high melting and boiling points. High density polyethene is a very common example of this type. Nylon, polyesters and PVC are also linear chain polymers.
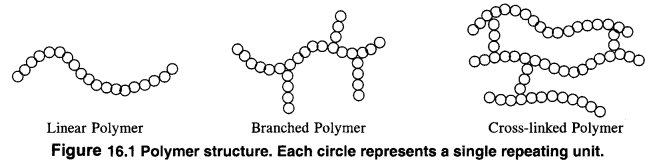
2. Branched chain polymers: In this type of polymers, the monomer units are linked to form long chains which have also side chains or branched chains of different Lengths attached to them. As a result of branching, these polymers are not closely packed in space. They have low densities, low tensile strength as well as low melting and boiling points. Some common examples of such polymers are ; low density polyethene, amylopectin, starch, glycogen etc.
3. Cross: linked polymers. In these polymers, also called net—work polymers, the monomer units are linked together to form three dimensionaL net—work as shown in the figure. These are expected to be quite hard, rigid and brittle. Examples of cross linked polymers are bakelite, glyptal. melamine formaldehyde polymer etc.
15.3. Write the names of the monomers of the following polymers:
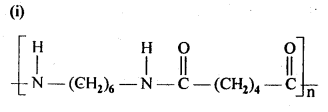
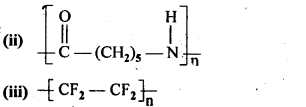
Ans: (i) Hexamethylene diamine NH2-(CH2)6NH2 and adipic acid HOOC – (CH2)4 – COOH
(ii) Caprolactum
(iii) Tetrafluoroethene F2C = CF2
15.4. Classify the following as addition and condensation polymers:
Terylene, Bakelite, Polyvinyl chloride,Polythene
Ans: Addition polymers: Polyvinyl chloride, Polythene
Condensation polymers : Terylene, bakelite.
15.5. Explain the difference between Buna-N and Buna-S.
Ans: Both Buna-N and Buna-S are synthetic rubber and are co-polymers in nature. They differ in their constituents.
Buna-N: Constituents are : buta-1, 3-diene and acrylonitrile.
Buna-S: Constituents are : buta-1, 3-diene, and styrene. They condense in the presence of Na.
Buna – S: It is a co—polymer of 1. 3 – butadiene and styrene and is prepared by the polymerisation of these components in the
ratio of 3 : 1 in the presence of sodium.
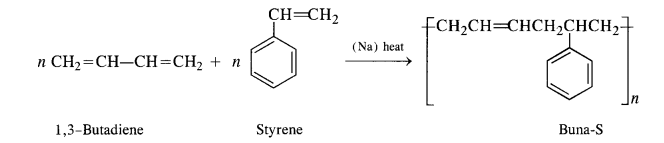
Buna-N (Nitrile rubber): h is a co-polymer of buta-1. 3-diene and acrylonitrile. It is formed as follows:
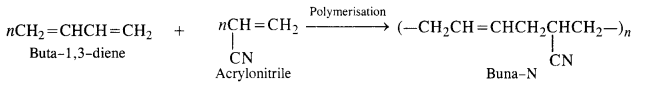
15.6. Arrange the following polymers in increasing order of their intermolecuiar forces.
(i) Nylon 6,6, Buna-S, Polythene
(ii) Nylon 6, Neoprene, Polyvinyl chloride
Ans: On the basis of intermolecuiar forces, polymers
are classified as elastomers, fibres and plastics. The increasing order of intermolecuiar forces is: Elastomer < Plastic < fibre.
Thus, we have
(i)Buns-S < Polythene < Nylon 6,6
(ii)Neoprene < Polyvinyl chloride < Nylon 6.
NCERT EXERCISES
15.1. Explain the terms polymer and monomer.
Ans: Polymers are high molecular mass substances consisting of a very large number of simple repeating structural units joined together through covalent bonds in a regular fashion. Polymers are also called macromolecules. Some examples are polythene, nylon-66, bakelite, rubber, etc. Monomers are the. simple and reactive molecules from which the polymers are prepared either by addition or condensation polymerisation. Some examples are ethene, vinyl chloride, acrylonitrile, phenol and formaldehyde etc.
15.2. What are natural and synthetic polymers ? Give two examples of each.
Ans:
1. Natural polymers: The polymers which occur in nature mostly in plants and animals are called natural polymers. A few common examples are starch, cellulose, proteins, rubber nucleic acids, etc. Among them, starch and cellulose are the polymers of glucose molecules. Proteins are formed from amino acids which may be linked in different ways. These have been discussed in detail in unit 15 on biomolecules. Natural rubber is yet another useful polymer which is obtained from the latex of the rubber tree. The monomer units are of the unsaturated hydrocarbon 2-methyl-i, 3-butadiene, also called isoprene.
Example of natural polymers: Natural rubber, cellulose, nucleic acids, proteins etc.
2. Synthetic polymers: The polymers which are prepared in the laboraroiy are called synthetic polymers. These are also called man made polymers and have been developed in the present century to meet the ever increasing demand of the modem civilisation.
Example of synthetic polymers: Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6 etc.
15.3. Distinguish between the terms homopolymer and copolymer and give an example of each.
Ans: Polymers whose repeating structural units are derived from only one type of monomer units are called homopolymers, e.g., PVC polyethene, PAN, teflon, polystyrene, nylon- 6 etc.
Polymers whose repeating structural units are derived from two or more types of monomer molecules are copolymers, e.g., Buna-S, Buna-N, nylon-66, polyester, bakelite.
15.4. How do you explain the functionality of a monomer?
Ans: Functionality of a monomer implies the number of bonding sites present in it. For example, monomers like propene, styrene, acrylonitrile have functionality of one which means that have one bonding site.
Monomers such as ethylene glycol, hexamethylenediamine, adipic acid have functionality of two which means that they have two bonding sites.
15.5. Define the term polymerisation?
Ans: It is a process of formation of a high molecular Sol. mass polymer from one or more monomers by linking together a large number of repeating structural units through covalent bonds.
15.6. Is (-NH — CHR—CO-)n a homopolymer or copolymer?
Ans: It is a homopolymer because the repeating structural unit has only one type of monomer, i.e., NH2—CHR—COOH.
15.7. In which classes, are the polymers classified on the basis of molecular forces?
Ans: Polymers are classified into four classes on the basis of molecular forces. These are:
elastomers, fibres, thermoplastic polymers and thermosetting polymers.
1. Elastomers: In these polymers, the intermolecular forces are the weakest. As a result, they can be readily stretched by applying small stress and regain their original shape when the stress is removed. The elasticity can be further increased by introducing some cross – links in the polymer chains. Natural rubber is the most popular example of elastomers. A few more examples are of: buna-S, buna-N and neoprene.
2. Fibres: Fibres represent a class of polymers which are thread-like and can be woven into fabrics in a number of ways. These are widely used for making clothes, nets, ropes, gauzes etc. Fibres possess high tensile strength because the chains possess strong intermolecular forces such as hydrogen bonding. These forces are also responsible for close packing of the chains. As a result, the fibres are crystalline in nature and have aJso sharp melting points. A few common polymers belonging to this class are nylon – 66, terylene and polyacrylonitrile etc.
3. Thermoplastics: These are linear polymers and have weak van der Waals forces acting in the various chains and are intermediate of the forces present in the elastomers and in the fibres. When heated, they melt and form a fluid which sets into a hard mass on cooling, Thus, they can be cast into different shapes by using suitable moulds. A few common examples are polyethene and polystyrene polyvinyls etc. These can be used for making toys, buckets, telephone apparatus, television cabinets etc.
4. Thermosetting plastics: These are normally semifluid substances with low molecular masses. When heated, they become hard and infusible due to the cross-linking between the polymer chains. As a result, they also become three dimensional in nature. They do not melt when heated. A few common thermosetting polymers are bakelite, melamine-formaldehyde, urea-formaldehyde and polyurethane etc.
15.8. How can you differentiate between addition and condensation polymerisatiop?
Ans: In addition polymerization, the molecules of the same or different monomers simply add on to one another leading to the formation of a macromolecules without elimination of small molecules like H2O, NH3 etc. Addition polymerization generally occurs among molecules containing double and triple bonds. For example, formation of polythene from ethene and neoprene from chloroprene, etc. In condensation polymerisation, two or more bifunctional trifimctional molecules undergo a series of independent condensation reactions usually with the elimination of simple molecules like water, alcohol, ammonia, carbon dioxide and hydrogen chloride to form a macromolecule. For example, nylon-6,6 is a condensation polymer of hexamethylenediamine and adipic acid formed by elimination of water molecules.
15.9. Explain the term copolymerisation and give two examples.
Ans: When two or more different monomers are allowed to polymerise together the product formed is called a copolymer, and the process in called copolymerisation. Example, Buna-S and Buna-N. Buna- S is a copolymer of 1, 3- butadiene and styrene while Buna-N is a copolymer of 1,3-butadiene and acrylonitrile.
15.10. Write the free radical mechanism for the polymerisation of ethene.
Ans:
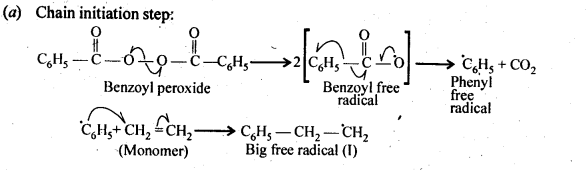
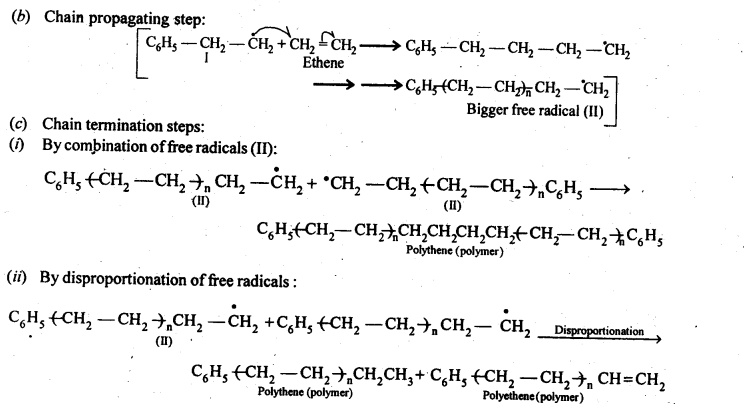
15.11. Define thermoplastics and thermo setting polymers with two examples of each
Ans: Thermoplastics polymers are linear polymer which can be repeatedly melted and moulded again and again on heating without any change in chemical composition and mechanical strength. Examples are polythene and polypropylene.
Thermosetting polymers, on the other hand, are permanently setting polymers. Once on heating in a mould, they get hardened and set, and then cannot be softened again. This hardening on heating is due to cross- linking between different polymeric chains to give a three dimensional network solid. Examples are bakelite, melamine-foimaldehyde polymer etc.
15.12. Write the monomers used for gettingThe following polymers:
(i) Polyvinylchloride
(ii) Teflon (iii) Bakelite
Ans:
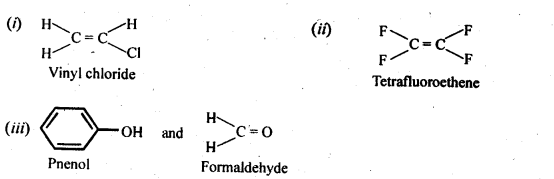
15.13. Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Ans:
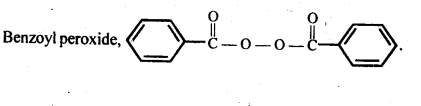
15.14. How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Ans: Natural rubber is cis-polyisoprene and is obtained by 1, 4-polymerization of isoprene units. In this polymer, double bonds are located between C2 and C3 of each isoprene unit. These cis-double bonds do not allow the polymer chains to come closer for effective interactions and hence intermolecular forces are quite weak. As a result, natural rubber, i.e., cis-polyisoprene has a randomly coiled structure not the linear one and hence show elasticity.
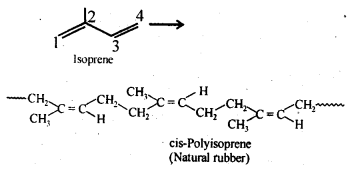
15.15. Discuss the main purpose of vulcanisation of rubber.
Ans: Natural rubber has the following disadvantages:
(a) It is soft and sticky and becomes even more so at high temperatures and brittle at low temperatures. Therefore, rubber is generally used in a narrow temperature range (283-335 K) where its elasticity is maintained.
(b)It has large water absorption capacity, has low tensile strength and low resistance to abrasion.
(c)It is not resistant to the action of organic solvents.
(d)It is easily attacked by oxygen and other oxidising agents. .
To improve all these properties, natural rubber is vulcanised by heating it with about 5% sulphur at 373-415 K. The vulcanized rubber thus obtained has excellent elasticity over a larger range of temperature, has low water absorption tendency and is resistant to the action of organic solvents and oxidising agents.
15.16. What are the monomeric repeating units of Nylon-6 and Nylon 6,6?
Ans:
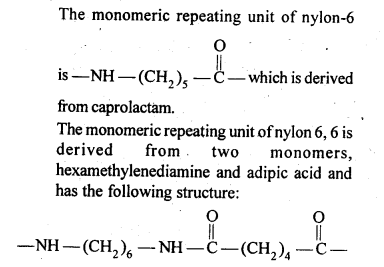
15.17. Write the names and structures of the monomers of the following polymers:
(i) Buna-S (ii) Buna-N (iii) Dacron (iv) Neoprene
Ans:
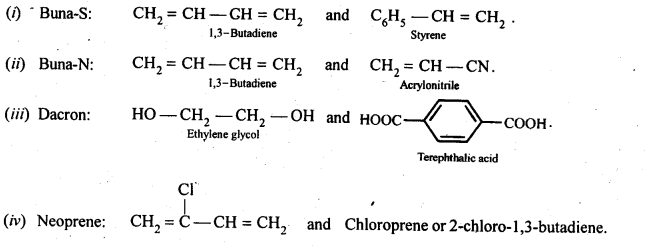
15.18. Identify the monomer in the following polymeric structures:
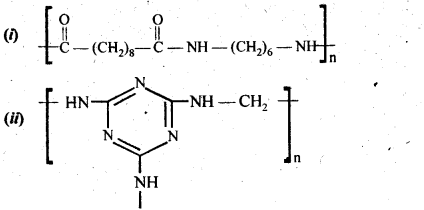
Ans:
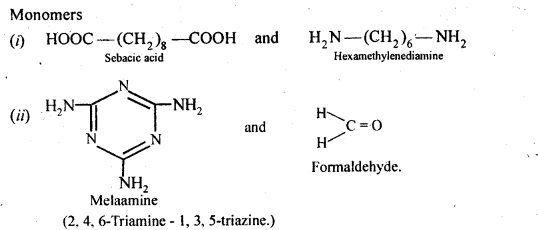
15.19. How is dacron obtained from ethylene glycol and terephthalic acid?
Ans: Dacron is obtained by condensation polymerization of ethylene glycol and terephthalic acid with the elimination of water molecules. The reaction is carried out at 420 – 460 K in presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide.
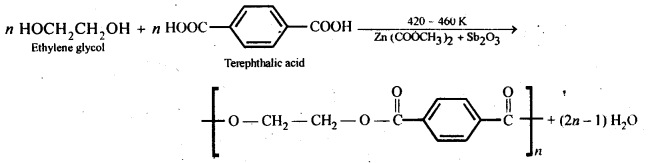
15.20. What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Ans: Polymers which disintegrate by themselves over a period of time due to environment degradation by bacteria, etc., are called biodegradable polymers. Example is PHBV, i. e., Poly-β-Hydroxybutyrate-co-β- Hydroxyvalerate.
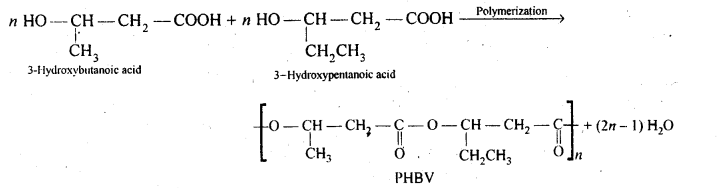
Class 12 Chemistry Chapter 16 Chemistry in Everyday Life:
Section Name
Topic Name
16
Chemistry in Everyday Life
16.1
Drugs and their Classification
16.2
Drug-Target Interaction
16.3
Therapeutic Action of Different Classes of Drugs
16.4
Chemicals in Food
16.5
Cleansing Agents
NCERT INTEXT QUESTIONS
16.1. Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor. Why?
Ans: Most of drugs taken in doses higher than recommended may produce harmful effects and act as poison and cause even death. Therefore, a doctor must always be consulted before taking the drug.
16.2. “Ranitidine is an antacid” With reference to which classification, has this statement been given?
Ans: Ranitidine is labelled as antacid since it is quite effective in neutralising the excess of acidity in the stomach. It is sold in the market under the trade name Zintac.
16.3. Why do we require artificial sweetening agents?
Ans: To reduce calorie intake and to protect teeth from decaying, we need artificial sweeteners.
16.4. Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structures of these compounds are given below:
(i) (C15H31COO)3C3H5-Glyceryl palmitate
(ii) (C17H32COO)3C3H5-Glyceryl oleate
Ans:
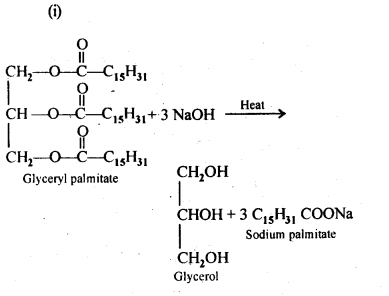
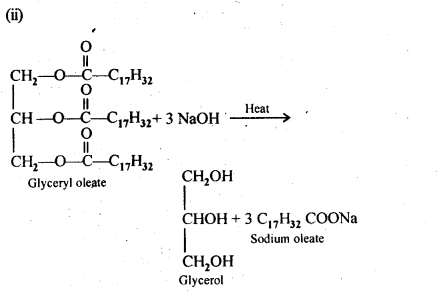
16.5. Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic part in the molecule. Identify the functional group (s) present in the molecule.
Ans:
Functional groups present in the detergent molecule are:
(i)ether
(ii)1°alcoholic group
NCERT EXERCISES
16.1. Why do we need to classify drugs in different ways?
Ans: Drugs are classified in following different ways:
(a) Based on pharmacological effect.
(b) Based on action on a particular biochemical process.
(c) Based on chemical structure.
(d) Based on molecular targets.
Each classification has its own usefulness.
(а) Classification based on pharmacological effect is useful for doctors because it provides them the whole range of drugs available for the treatment of a particular disease.
(b) Classification based on action on a particular biochemical proc*ess is useful for choosing the correct compound for designing the synthesis of a desired drug.
(c) Classification based on chemical structure helps us to design the synthesis of a number of structurally similar compounds having different substituents and then choosing the drug having least toxicity.
(d) Classification on the basis of molecular targets is useful for medical chemists so that they can design a drug which is most effective for a particular receptor site.
16.2. Explain the following as used in medicinal chemistry
(a) Lead compounds
(b) Target molecules or drug targets.
Ans:
(a) Lead compounds are the compounds which are effective in different drugs. They have specific chemical formulas and may be extracted either from natural sources (plants and animals) or may be synthesised in the laboratory.
(b) Target molecules or drug targets. An enzyme (E) functions by combining with the reactant (called substrate) denoted as ‘S’ to form an activated complex known as enzyme-substrate complex (E-S). The complex dissociates to form product and releases the enzyme for carrying out further activity.
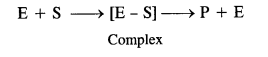
16.3. Name the macro molecules that are chosen as drug targets.
Ans: Proteins, carbohydrates, lipids and nucleic acids are chosen as drug targets.
16.4. Why the medicines should not be taken without consulting doctors?
Ans: No doubt medicines are panacea for most of the body ailments. But their wrong choice and overdose can cause havoc and may even prove to be fatal. Therefore, it is of utmost importance that the medicines should not be given without consulting doctors.
16.5. Define the term chemotherapy.
Ans: It is the branch of chemistry that deals with the treatment of diseases by using chemicals as medicines.
16.6. Which forces are involved in holding the drugs to the active site of enzymes?
Ans: The following forces are involved in holding the drugs to the active site of enzymes:
(a) Hydrogen bonding
(b) Ionic bonding
(c) Dipole-dipole interactions
(d) van der Waals interactions
16.7. Antacids and antiallergic drugs interfere with the function of histamines but do not interfere with the function of each other. Explain.
Ans: They donot interfere with the functioning of each other because they work on different receptors in the body.Histamine stimulates the secretion of pepsin and hydrochloric acid in the stomach. The drug cimetidine (antacid) was designed to prevent the interaction of histamine with the receptors present in the stomach wall. This resulted in release of lesser amount of acid. Antacid and antiallergic drugs work on different receptors.
16.8. Low level of noradrenaline is the cause of depression. What type of drugs are needed to cure this problem? Name two drugs.
Ans: In case of low level of neurotransmitter, . noradrenaline, tranquilizer (antidepressant) drugs are required because low levels of noradrenaline leads to depression. These drugs inhibit the enzymes which catalyse the degradation of noradrenaline. If the enzyme is inhibited, noradrenaline is slowly metabolized and can activate its receptor for longer periods of time thereby reducing depression. Two important drugs are iproniazid and phenylzine.
16.9. What is meant by the term broad spectrum antibiotics? Explain.
Ans: Broad spectrum antibiotics are effective against several different types or wide range of harmful bacteria. For example, tetracycline, chloramphenicol and of loxacin. Chloramphenicol can be used in case of typhoid, dysentry, acute fever, urinary infections, meningitis and pneumonia.
16.10. How do antiseptics differ from disinfectants ? Give one example of each.
Ans: Many times, the same substance can act as an antiseptic as well as disinfectant by changing the concentration of the solution used. For example, a 0.2 per cent solution of phenol acts as an antiseptic and its 1 percent solution is a disinfectant. Chlorine is used in India for making water fit for drinking at a concentration of 0.2 to 0.4 ppm (parts per million). Low concentration of sulphur dioxide is used for sterilizing squashes for preservation. A few points of distinction between antiseptics and disinfectants are listed.
Antiseptics
Disinfectants
1. Can kill or prevent the growth of micro-organisms.
1.Can kill micro-organisms.
2. Do not harm the living tissues. Therefore, these can be applied to the skin.
2. Toxic to the living tissues. Therefore, these cannot be applied to the skin.
3. These are used for the dressing of wounds, ulcers and in the treatment of diseased skin.
3. These are used for disinfecting floors, toilets, drains, instruments etc.
16.11. Why are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide?
Ans: If excess of NaHCO3 or Mg(OH)2 or Al(OH)3 is used, it makes the stomach alkaline and thus triggers the release of even more HCl which may cause ulcer in the stomach. In contrast, cimetidine and ranitidine prevent the interaction of histamine with the receptor cells in the stomach wall and thus release of HCl will be less as histamine stimulates the secretion of acid.
16.12. Name a substance which can be used as an antiseptic as well as disinfectant.
Ans: 0.2% solution of phenol acts as antiseptic while 1% solution acts as a disinfectant.
16.13. What are the main constituents of dettol?
Ans: Chloroxylenol .and α-terpineol in a suitable solvent.
16.14. What is tincture of iodine? What is its use?
Ans: 2-3% solution of iodine in alcohol and water is called tincture of iodine. It is a powerful antiseptic. It is applied on wounds.
16.15. What are food preservatives?
Ans: Preservation has a major role in the food products. Chemically preserved squashes and crushes can be kept for a fairly long time even after opening the seal of bottle.
A preservative may be defined as the substance which is capable of inhibiting or arresting the process of fermentation, acidification or any other decomposition of food. Salting i.e. addition of table salt is a well known method for food preservation and was applied in ancient times for preserving raw mangoes, tamarind, meat, fish etc. Sugar syrup can also act as a preservative. Vinegar is a useful preservative for pickles. Apart from these, sulphur dioxide and benzoic acid can be employed for the preservation of food. The major source of sulphur dioxide is potassium metabisulphite (K2S2O5). It is fairly stable in neutral and alkaline medium but gets decomposed by weak acids such as carbonic, citric, tartane and malic acids. Benzoic acid is used either as such or in the form of sodium benzoate. However, sulphur dioxide has a better preservative action than sodium benzoate against bacteria and moulds. It also retards the development of yeast in juice but fails to arrest their multiplication once the number has reached a high value. Sorne salts of sorbic acid and propionic acid are also being used these days for the preservation of the food.
The use of preservatives must be properly controlled as their indiscriminate use is likely to be harmful. The preservative should not be injurious to health and should be also non-irritant.
16.16. Why is the use of aspartame limited to cold foods and drinks?
Ans: This is because it decomposes at baking or cooking temperatures and hence can be used only in cold foods and drinks as an artificial sweetener
16.17. What are artificial sweetening agents? Give two examples.
Ans: Artificial sweeteners are chemical substances which are sweet in taste but do not add any calories to our body. They are excreted as such through urine. For example, saccharin, aspartame, alitame etc.
16.18. Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Ans: Saccharine, aspartame or alitame may be used in the preparation of sweets for a diabetic patient.
16.19. What problem arises in using alitame as artificial sweetener?
Ans: Alitame is a high potency artificial sweetener.Therefore, it is difficult to control the sweetness of the food to which it is added.
16.20. How are synthetic detergents better than soaps?
Ans: They can be used in hard water as well as in acidic solution. The reason being that sulphonic acids and their calcium and magnesium salts are soluble in water thus they do not form curdy white precipitate with hard water but the fatty acids and their calcium and magnesium salts of soaps are insoluble. Detergents also works in slightly acidic solution due to formation of soluble alkyl hydrogen sulphates. Soaps react with acidic solution to form insoluble fatty acids.
16.21. Explain the following terms with suitable examples:
(i) cationic detergents (ii) anionic detergents and (iii) non-ionic detergents
Ans: (i) Cationic detergents: These are quaternary ammonium salts, chlorides, acetates, bromides etc containing one or more long chain alkyl groups. For example, cetyltrimethyl ammonium chloride.
(ii) Anionic detergents are called so because a large part of their molecules are anions. ‘These are of two types:
(a) Sodium alkyl sulphates: For example, sodium lauryl sulphate, C11H23CH2OSO3 Na+.
(b) Sodium alkylbenzenesulphonates.Vor example, sodium 4-(l-dodecyl) benzenesu Iphphonate (SDS).
(iii) Neutral or non-ionic detergents: These are esters of high molecular mass alcohols with fatty acids. These can also be obtained by treatment of long chain alcohols by with excess of ethylene oxide in presence of a base. For example, polyethylene glycol stearate,CH3(CH2)16COO (CH2CH2O)11 CH2CH2OH Polyethylene glycol stearate.
16.22. What are biodegradable and non-biodegradable detergents? Give one example of each.
Ans: Detergents having straight chain hydrocarbons are easily degraded (or decomposed) by microorganisms and hence are called biodegradable detergents while detergents containing branched hydrocarbon chains are not easily degraded by the microorganisms find hence are called non-biodegradable detergents. Consequently, non-biodegradable detergents accumulate in rivers and water ways thereby causing severe water pollution. Examples of biodegradable detergents are sodium lauryl sulphate, sodium 4-(-l-dodecyl) benzenesulphonate and sodium 4-(2-dodecyl) benzenesulphonate.
Examples of non-biodegradable detergents is sodium 4-(1, 3,5,7 – tetramethyloctyl) benzene sulphonate.
16.23. Why do soaps not work in hard water? (C.B.S.E. Outside Delhi 2009, 2011)
Ans: Soaps are water soluble sodium or potassium salts of higher fatty acids like palmitic acid (C15H31COOH), oleic acid (C17H33COOH) and stearic acid (C17H35COOH). Hard water contains certain calcium and magnesium salts which combine with soaps to form corresponding magnesium compounds. These being insoluble, get separated as curdy white precipitates resulting in wastage of soap.
16.24. Can you use soaps and synthetic detergents to check the hardness of water?
Ans: Soaps get precipitated as insoluble calcium and magnesium soaps in hard water but detergents do not. Therefore, soaps but not synthetic detergents can be used to check the hardness of water.
16.25. Explain the cleansing action of soaps.
Ans: Cleansing action of soaps : Soaps contain two parts, a large hydrocarbon which is a hydrophobic (water repelling) and a negative charged head, which is hydrophillic (water attracting). In solution water molecules being polar in nature, surround the ions & not the organic part of the molecule. When a soap is dissolved in water the molecules gather together as clusters, called micelles. The tails stick inwards & the head outwards. The hydrocarbon tail attaches itself to oily dirt. When water is agitated, the oily dirt tends to lift off from the dirty surface & dissociates into fragments. The solution now contains small globules of oil surrounded detergent molecules. The negatively charged heads present in water prevent the small globules from coming together and form aggregates. Thus the oily dirt is removed from the object.
16.26. If water contains dissolved calcium hydrogencarbonate, out of soaps and synthetic detergents, which one will you use for cleaning clothes?
Ans: Calcium hydrogencarbonate makes water hard. Therefore, soap cannot be used because it gets precipitated in hard water. On the other hand, a synthetic detergent does not precipitate in hard water because its calcium salt is also soluble in water. Therefore, synthetic detergents can be used for cleaning clothes in hard water.
16.27. Label the hydrophilic and hydrophobic parts in the following compounds.
(i)cCH3(CH2)10CH2OSO3 –Na+
(ii) CH3(CH2)15 -N+(CH3)3Br–
(iii) CH3(CH2)16C00(CH2CH2O)11CH2CH2OH
Ans:
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules:
| Section Name | Topic Name |
| 14 | Biomolecules |
| 14.1 | Carbohydrates |
| 14.2 | Proteins |
| 14.3 | Enzymes |
| 14.4 | Vitamins |
| 14.5 | Nucleic Acids |
| 14.6 | Hormones |
NCERT INTEXT QUESTIONS
14.1. Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain.
Ans: The .solubility of a solute in a given solvent follows the rule ‘ Like dissolves like’.Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water. As a result of this extensive intermoleeular H-bonding, glucose and sucrose are soluble in water.On the other hand, benzene and cyclohexane do not contain -OH bonds and hence do not form H-bonds with water. Moreover, they are non-polar molecules and hence do not dissolve in polar water molecules.
14.2. What are the expected products of hydrolysis of lactose?
Ans: Lactose being a disaccharide gives two molecules of monosaccharides Le. one molecule each of D-(+) – glucose and D-(+)-galactbse.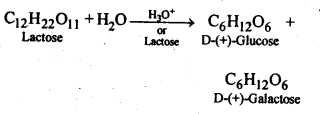
14.3. How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Ans: The cyclic hemiacetal form of glucose contains an -OH group at C-l which gets hydrolysed in aqueous solution to produce open chain aldehydic form which then reacts with NH2OH -to form corresponding oxime. Thus, glucose contains an aldehydic group. However, when glucose is reacted with acetic anhydride, the -OH group at C-l along with the other -OH groups at C-2, C-3, C-4 and C-6 form a pentaacetate.
Since the penta acetate of1 glucose does not contain a free -OH group at C-l, it cannot get hydrolysed in aqueous solution to produce open chain aldehydic form and hence glucose pentaacetate does not react with NH2OH to form glucose oxime. The reactions are shown as:
14.4. The melting points and solubility in water of a-amino acids are generally higher than those of corresponding haloacids. Explain.
Ans: a-amino acids as we all know, are dipolar in nature (
14.5. Where does the water present in the egg go after boiling the egg?
Ans: When egg is boiled, proteins first undergo denaturation and then coagulation and the water present in the egg gets absorbed in coagulated protein, probably through H- bonding
14.6. Why cannot Vitamin C be stored in our body?
Ans: Vitamin C cannot be stored in the body because it is water soluble and is, therefore, easily excreted in urine.
14.7. Which products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Ans: Upon hydrolysis, nucleotide from DNA would form 2-deoxyribose and phosphoric acid along-with thymine.
14.8. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Ans: A DNA molecule has two strands in which the four complementary bases pair each other, i.e., cytosine (C) always pair with guanine (G) while thymine (T) always pairs with adenine (A). Thus, when a DNA molecule is hydrolysed, the molar amounts of cytosine is always equal to that of guanine and that of adenine is always equal to thymine.In RNA, there is no relationship between the quantities of four bases (C, G, A and U) obtained, therefore, the base pairing principle, i.e. A pairs with U and C pairs with G is not followed. Therefore, unlike DNA, RNA has a single strand.
NCERT EXERCISES
14.1. What are monosaccharides ?
Ans: Monosaccharides are carbohydrates Which cannot be hydrolysed to smaller molecules.Their general formula is (CH2O)n Where n=3-7 These are of two types: Those which contain an aldehyde group (-CHO) are called aldoses and those which contain a keto (C=O) group are called ketoses.
They are further classified as trioses , tetroses ,pentoses , hexoses and heptoses according as they contain 3,4,5,6, and 7 carbon atoms respectively.For example.
14.2. What are reducing sugars?
Ans: Reducing sugars are those which can act as reducing agents. They contain in them a reducing group which may be aldehydic (-CHO) or ketonic (>C=0) group. The characteristic reactions of reducing sugars are with Tollen’s reagent and Fehling solution. Non-reducing sugars donot give these reactions. For example, glucose, fructose, lactose etc. are reducing sugars. Sucrose is regarded as a non-reducing sugar because both glucose and fructose are linked through their aldehydic and ketonic groups by glycosidic linkage. Since these groups are not free, sucrose is a non-reducing sugar.
14.3. Write two main functions of carbohydrates in plants.
Ans: Two major functions of carbohydrates in plants are following
(a)Structural material for plant cell walls: The polysaccharide cellulose acts as the chief structural material of the plant cell walls.
(b)Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and act as the reserve food material for the tiny plant till it is capable of making its own food by photosynthesis.
14.4. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans: Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose. Disaccharides: Maltose and lactose.
14.5. What do you understand by the term glycosidic linkage?
Ans: The ethereal or oxide linkage through which two monosaccharide units are joined together by the loss of a water molecule to form a molecule of disaccharide is called the glycosidic linkage. The glycosidic linkage in maltpse molecule is shown below:
14.6. What is glycogen? How is it different from starch?
Ans: The carbohydrates are stored in animal body as glycogen. It is also called animal starch and its structure is similar toamylopectin which means that it is a branched chain polymer of α-D-glucose units in which the chain is formed by C1 – C4 glycosidic linkage whereas branching occurs by the formation of C1– C6 glycosidic linkage. One main difference between glycogen and amylopectin is the length of the chain. In amylopectin, the chain consists of 20 – 25 α – D – glucose molecules whereas in glycogen, there are 10 -14 molecules of α – D – glucose present. Glycogen is more branched than amylopectin. It is present mainly in liver, muscles and also in brain. Glycogen gets converted into glucose when the body needs it with the help of certain enzymes present in the body. Glycogen has also been found to be present in yeast and fungi.
Starch is a major source of carbohydrates which are very much essential to the human body since they supply energy to the body. It occurs as granules mainly in seeds, fruits, tubers and also in the roots of the plants. The chief commercial sources of starch are wheat, maize, rice, potatoes etc.
14.7. What are the hydrolysis products of (i) sucrose, and (ii) lactose?
Ans: Both sucrose and lactose are disaccharides. Sucrose on hydrolysis gives one molecule each of glucose and fructose but lactose on hydrolysis gives one molecule each of glucose and galactose.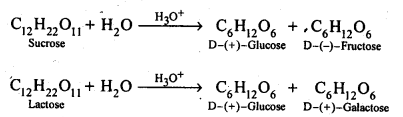
14.8. What is the basic structural difference between starch and cellulose?
Ans: Starch consists of amylose and amylopectin. Amylose is a linear polymer of α-D-glucose while cellulose is a linear polymer of β -D- glucose. In amylose, C -1 of one glucose unit is connected to C – 4 of the other through α-glycosidic linkage. However in cellulose, C – 1 of one glucose unit is connected to C-4 of the other through β – glycosidic linkage. Amylopectin on the other hand has highly branched structure.
14.9. What happens when D-glucose is treated with . the following reagents.
(i) HI
(ii) Bromine water
(iii) HNO3
Ans:
14.10. Enumerate the reactions of D-glucose which cannot be explained with open chain structure. (C.B.S.E. Delhi 2008, C.B.S.E. Sample Paper 2011)
Ans:
Open chain structure of D-glucose contains a free aldehydic group (- CHO). However, it does not give the following reactions:
- D(+) glucose does not react with 2, 4 D.N.P.
- D(+) glucose does not react with NaHSO3.
- D(+) glucose does not restore the pink colour to Schiff’s reagent.
- Penia acetyl glucose formed by carrying acetylation with acetic anhydride does not react with hydroxyl amine
(NH2OH) which is the characteristic reaction of all aldehydes. - D( +) glucose is found to exist in two different crystalline forms which are named as α and β. Both these forms have actually been isolated. For example, α form with m.p. 419 K is obtained by the crystallisation of the saturated solution of glucose prepared at 303 K. Similarly, β-form with m.p. 423 K is isolated by carrying out the crystallisation of the saturated solution of glucose prepared at 371 K. Apart from that the a-form has a specific rotation (α) equal to + 112° while the β- form has specific rotation (α) equal to + 19°.
In the light of the limitations stated above, Tollen stated that an open chain structure for D(+) glucose is probably not practicable. He proposed a cyclic structure which is a hemiacetal structure. In this structure, the aldehydic (CHO) group
is involved in the form of a ring with the -OH group attached to C5 carbon. It is a six membered ring, often called ô-
oxide ring. The ring structure accounts for the two isomeric forms a and shown below.
14.11. What are essential and non-essential amino acids? Give two examples of each type.
Ans: α-Amino acids which are needed for good health and proper growth of human beings but are not synthesized by the human body are called- essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, α-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid etc.
14.12. Define the following as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
Ans: (i) Peptide bond: Proteins are condensation polymers of α-amino acids in which the same or different α-amino acids are joined by peptide bonds. Chemically, a peptide bond is an amide linkage formed between – COOH group of one α-amino acid and -NH-, group of the other α-amino acid by lo;ss of a molecule of water. For example,
(ii) Primary structure: Proteins may contain one or more polypeptide chains. Each . polypeptide chain has a large number of α-amino acids which are linked to one another in a specific manner. The specific sequence in which the various amino acids present in a protein linked to one another is called its primary structure. Any change in the sequence of α-amino acids creates a different protein.
(iii) Denaturation: Each protein in the biological system has a unique three-dimensional structure and has specific biologicalactivity. This is called native form of a protein. When a protein in its native form is subjected to a physical change such as change in temperature or a chemical change like change in pH, etc., hydrogen bonds gets broken. As a result, soluble forms of proteins such as globular proteins undergo coagulation or precipitation to give fibrous proteins which are insoluble in water. This coagulation also results in loss of biological activity of the proteins and this loss in biological activity, is called denaturation. During denaturation, 2° and 3° structures of proteins are destroyed but 1° structure remains intact.
The most common example of denaturation of proteins is the coagulation of albumin present in the white of an egg. When the egg is boiled hard, the soluble globular protein present in it is denatured and is converted into insoluble fibrous protein.
14.13. What are the common types of secondary structure of proteins?
Ans:
Secondary structure of protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
- α-helix structure
- β-pleated sheet structure.
Secondary Structure of Proteins:
The long, flexible peptide chains of proteins are folded into the relatively rigid regular conformations called the
secondary structure. It refers to the conformation which the polvpeptide chains assume as a result of hydrogen bonding
between the > C= O and > N-H groups of different peptide bonds.
The type of secondary structure a protein will acquire, in general depends upon the size of the R-group. If the size of the
R-groups are quite large, the protein will acquire ct-helix structure. If on the other hand, the size of the R-groups are relatively
smaller, the protein will acquire a β – flat sheet structure.
(a) α-Helix structure: If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group
of one amino acid unit and the > N-H group of the fourth amino acid unit within the same chain. As such the polypeptide
chain coils up into a spiral structure called right handed ct- helix structure. This type of structure is adopted by most of the
fibrous structural proteins such as those present in wool, hair and muscles. These proteins are elastic i.e., they can be
stretched. During this process, the weak hydrogen bonds causing the a – helix are broken. This tends to increase the length of
the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.
(b) β—Flat sheet or β—Pleated sheet structure: If R-groups are relatively small, the peptide chains lie side by side in a zig
zag manner with alternate R-groups on the same side situated at fixed distances apart. The two such neighbouring chains are held together by intermolecular hydrogen bonds. A number of such chains can be inter-bonded and this results in the formation of a flat sheet structure These chains may contract or bend a little in order to accommodate moderate sized R-groups. As a result, the sheet bends into parallel folds to form pleated sheet structure known as β – pleated sheet structure. These sheets are then stacked one above the other like the pages of a book to form a three dimensional structure. The protein fibrion in silk fibre has a β – pleated sheet structure. The characteristic mechanical properties of silk can easily be explained on the basis of its β – sheet structure. For example, silk is non-elastic since stretching leads to pulling the peptide covalent bonds. On the other hand, it can be bent easily like a stack of pages because during this process, the sheets slide over each other.
14.14. What types of bonding helps in stabilising the α-helix structure of proteins?
Ans: α-helix structure of proteins is stabilised through hydrogen bonding. (a) α -Helix structure. If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group of one amino acid unit and the > N- H group of the fourth amino acid unit within the same chain. As such the polypeptide chain coils up into a spiral structure called right handed a—helix structure. This type of structure is adopted by most of the fibrous structural proteins such as those present in wool, hair and muscles. These proteins are elastic i.e., they can be stretched. During this process, the weak hydrogen bonds causing the α-helix are broken. This tends to increase the length of the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.
14.15: Differentiate between globular and fibrous proteins.
Ans. (i) Fibrous proteins: These proteins consist of linear thread like molecules which tend to lie side by side (parallel) to form fibres. The polypeptide chains in them are held together usually at many points by hydrogen bonds and some disulphide bonds. As a result,intermolecular forces of attraction are very’ strong and hence fibrous proteins are insoluble in water. Further, these proteins are stable to moderate changes in temperature and pH. Fibrous proteins serve as the chief structural material of animal tissues.For example, keratin in skin, hair, nails and wool, collagen in tendons, fibrosis in silk and myosin in muscles.
(ii) Globular proteins: The polypeptide chain in these proteins is folded around itself in such a way so as to give the entire protein molecule an almost spheroidal shape. The folding takes place in such a manner that hydrophobic (non-polar) parts are pushed inwards and hydrophilic (polar) parts are pushed outwards. As a result, water molecules interact strongly with the polar groups and hence globular protein are water soluble. As compared to fibrous proteins, these are very sensitive to small changes of temperature and pH. This class of proteins include all enzymes, many hormones such as insulin from pancreas, thyroglobulin from thyroid gland, etc.
14.16. How do you explain the amphoteric behaviour of amino acids?
Ans: Amino acids contain an acidic (carboxyl group) and basic (amino group) group in the same molecule. In aqueous solution, they neutralize each other. The carboxyl group loses a proton while the amino group accepts it. As a result, a dipolar or zwitter ion is formed.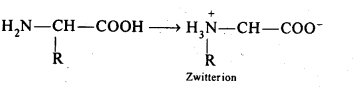
In zwitter ionjc form, a-amino acid show amphoteric behaviour as they react with both acids and bases.
14.17. What are enzymes?
Ans: Enzymes are biological catalyst. Each biological reaction requires a different enzyme. Thus, as compared to conventional catalyst enzymes are very specific and efficient in their action. Each type of enzyme has its own specific optimum conditions of concentration, pH and temperature at which it works best.
14.18. What is the effect of denaturation on the structure of proteins?
Ans: Denaturation of proteins is done either by change in temperature (upon heating) or by bringing a change in the pH of the medium. As a result, the hydrogen bonding is disturbed and the proteins lose their biological activity i.e., their nature changes. During the denaturation, both the tertiary and secondary structures of proteins are destroyed while the primary structures remain intact.
14.19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans: Vitamins are classified into two groups depending upon their solubility in water or fat: (i) Water soluble vitamins: These include vitamin B-complex (B1, B2, B5, i.e., nicotinic acid,B6, B12, pantothenic acid, biotin, i.e., vitamin H and folic acid) and vitamin C.
(ii) Fat soluble vitamins: These include vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues. Vitamin K is responsible for coagulation of blood.
14.20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Ans: Vitamin A is essential for us because its deficiency causes xerophthalmia (hardening of cornea of eye) and night blindness.
Sources: Fish liver oil, carrots, butter, milk, etc. Vitamin C is essential for us because its deficiency causes scurvy (bleeding of gums) and pyorrhea (loosening and bleeding of teeth). Sources: Citrous fruits, amla, green leafy vegetables etc.
14.21. What are nucleic acids ? Mention their two important functions.
Ans: Nucleic acids are biomolecules which are found in the nuclei of all living cell in form of nucleoproteins or chromosomes (proteins contains nucleic acids as the prosthetic group).
Nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid.(RNA).
The two main functions of nucleic acids are:
(a) DNA is responsible for transmission of hereditary effects from one generation to another. This is due to its unique property of replication, during cell division and two identical DNA strands are transferred to the daughter cells.
(b) DNA and RNA are responsible for synthesis of all proteins needed for the growth and maintenance of our body. Actually the proteins are synthesized by various RNA molecules (r-RNA, m-RNA) and t-RNA) in the cell but the message for the synthesis of a particular protein is coded in DNA.
14.22. What is the difference between a nucleoside and a nucleotide?
Ans: A nucleoside contains only two basic components of nucleic acids i.e., a pentose sugar and a nitrogenous base. It is formed when 1- position of pyrimidine (cytosine, thiamine or uracil) or 9-position of purine (guanine or adenine) base is attached to C -1 of sugar (ribose or deoxyribose) by a β-linkage. Nucleic acids are also called polynucleotides since the repeating structural unit of nucleic acids is a nucleotide.
A nucleotide contains all the three basic . components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and a nitrogenous base. These are obtained by esterification of C5, – OH group of the pentose sugar by phosphoric acid.
14.23. The two strands in DNA are not identical but are complementary. Explain.
Ans: The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (cytosine) through three H-bonds, (i.e.,C = G) and between A (adenine) and T (thiamine) through two H-bonds (i.e., A = T). Due to this base -pairing principle, the sequence of bases in one strand automatically fixes the sequence of bases in the other strand. Thus, the two strands are complimentary and not identical.
14.24. Write the important structural and functional differences between DNA and RNA.
Ans:
14.25. What are the different types of RNA found in the cell?
Ans: There are three types of RNA:
(a) Ribosomal RNA (r RNA)
(b) Messenger RNA (m RNA)
(c) Transfer RNA (t RNA)
Class 12 Chemistry Chapter 15 Polymers:
| Section Name | Topic Name |
| 15 | Polymers |
| 15.1 | Classification of Polymers |
| 15.2 | Types of Polymerisation Reactions |
| 15.3 | Molecular Mass of Polymers |
| 15.4 | Biodegradable Polymers |
| 15.5 | Polymers of Commercial Importance |
NCERT INTEXT QUESTIONS
15.1. What are polymers?
Ans: Polymers are high molecular mass substances (103 — 107u) consisting of a very large number of simple repeating structural units joined together through covalent bonds in a linear fashion. They are also called macromolecules. Ex: polythene, nylon 6,6, bakelite, rubber, etc.
15.2. How are polymers classified on the basis of structure?
Ans: On the basis of structure, polymers are classified into three types. These are linear chain polymers, branched chain polymers and crossedlinkedpolymers.
1. Linear chain polymers: In this case, the monomer units are linked to one another to form long linear chains. These linear chains are placed one above the other and are closely packed in space. The close packing results in high densities, tensile strength and also high melting and boiling points. High density polyethene is a very common example of this type. Nylon, polyesters and PVC are also linear chain polymers.
2. Branched chain polymers: In this type of polymers, the monomer units are linked to form long chains which have also side chains or branched chains of different Lengths attached to them. As a result of branching, these polymers are not closely packed in space. They have low densities, low tensile strength as well as low melting and boiling points. Some common examples of such polymers are ; low density polyethene, amylopectin, starch, glycogen etc.
3. Cross: linked polymers. In these polymers, also called net—work polymers, the monomer units are linked together to form three dimensionaL net—work as shown in the figure. These are expected to be quite hard, rigid and brittle. Examples of cross linked polymers are bakelite, glyptal. melamine formaldehyde polymer etc.
15.3. Write the names of the monomers of the following polymers: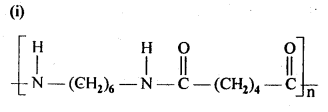
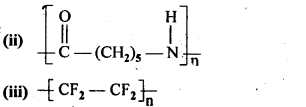
Ans: (i) Hexamethylene diamine NH2-(CH2)6NH2 and adipic acid HOOC – (CH2)4 – COOH
(ii) Caprolactum
(iii) Tetrafluoroethene F2C = CF2
15.4. Classify the following as addition and condensation polymers:
Terylene, Bakelite, Polyvinyl chloride,Polythene
Ans: Addition polymers: Polyvinyl chloride, Polythene
Condensation polymers : Terylene, bakelite.
15.5. Explain the difference between Buna-N and Buna-S.
Ans: Both Buna-N and Buna-S are synthetic rubber and are co-polymers in nature. They differ in their constituents.
Buna-N: Constituents are : buta-1, 3-diene and acrylonitrile.
Buna-S: Constituents are : buta-1, 3-diene, and styrene. They condense in the presence of Na.
Buna – S: It is a co—polymer of 1. 3 – butadiene and styrene and is prepared by the polymerisation of these components in the
ratio of 3 : 1 in the presence of sodium.
Buna-N (Nitrile rubber): h is a co-polymer of buta-1. 3-diene and acrylonitrile. It is formed as follows: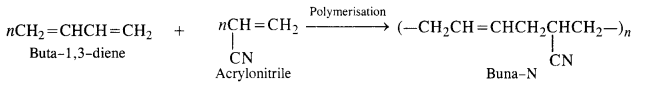
15.6. Arrange the following polymers in increasing order of their intermolecuiar forces.
(i) Nylon 6,6, Buna-S, Polythene
(ii) Nylon 6, Neoprene, Polyvinyl chloride
Ans: On the basis of intermolecuiar forces, polymers
are classified as elastomers, fibres and plastics. The increasing order of intermolecuiar forces is: Elastomer < Plastic < fibre.
Thus, we have
(i)Buns-S < Polythene < Nylon 6,6
(ii)Neoprene < Polyvinyl chloride < Nylon 6.
NCERT EXERCISES
15.1. Explain the terms polymer and monomer.
Ans: Polymers are high molecular mass substances consisting of a very large number of simple repeating structural units joined together through covalent bonds in a regular fashion. Polymers are also called macromolecules. Some examples are polythene, nylon-66, bakelite, rubber, etc. Monomers are the. simple and reactive molecules from which the polymers are prepared either by addition or condensation polymerisation. Some examples are ethene, vinyl chloride, acrylonitrile, phenol and formaldehyde etc.
15.2. What are natural and synthetic polymers ? Give two examples of each.
Ans:
1. Natural polymers: The polymers which occur in nature mostly in plants and animals are called natural polymers. A few common examples are starch, cellulose, proteins, rubber nucleic acids, etc. Among them, starch and cellulose are the polymers of glucose molecules. Proteins are formed from amino acids which may be linked in different ways. These have been discussed in detail in unit 15 on biomolecules. Natural rubber is yet another useful polymer which is obtained from the latex of the rubber tree. The monomer units are of the unsaturated hydrocarbon 2-methyl-i, 3-butadiene, also called isoprene.
Example of natural polymers: Natural rubber, cellulose, nucleic acids, proteins etc.
2. Synthetic polymers: The polymers which are prepared in the laboraroiy are called synthetic polymers. These are also called man made polymers and have been developed in the present century to meet the ever increasing demand of the modem civilisation.
Example of synthetic polymers: Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6 etc.
15.3. Distinguish between the terms homopolymer and copolymer and give an example of each.
Ans: Polymers whose repeating structural units are derived from only one type of monomer units are called homopolymers, e.g., PVC polyethene, PAN, teflon, polystyrene, nylon- 6 etc.
Polymers whose repeating structural units are derived from two or more types of monomer molecules are copolymers, e.g., Buna-S, Buna-N, nylon-66, polyester, bakelite.
15.4. How do you explain the functionality of a monomer?
Ans: Functionality of a monomer implies the number of bonding sites present in it. For example, monomers like propene, styrene, acrylonitrile have functionality of one which means that have one bonding site.
Monomers such as ethylene glycol, hexamethylenediamine, adipic acid have functionality of two which means that they have two bonding sites.
15.5. Define the term polymerisation?
Ans: It is a process of formation of a high molecular Sol. mass polymer from one or more monomers by linking together a large number of repeating structural units through covalent bonds.
15.6. Is (-NH — CHR—CO-)n a homopolymer or copolymer?
Ans: It is a homopolymer because the repeating structural unit has only one type of monomer, i.e., NH2—CHR—COOH.
15.7. In which classes, are the polymers classified on the basis of molecular forces?
Ans: Polymers are classified into four classes on the basis of molecular forces. These are:
elastomers, fibres, thermoplastic polymers and thermosetting polymers.
1. Elastomers: In these polymers, the intermolecular forces are the weakest. As a result, they can be readily stretched by applying small stress and regain their original shape when the stress is removed. The elasticity can be further increased by introducing some cross – links in the polymer chains. Natural rubber is the most popular example of elastomers. A few more examples are of: buna-S, buna-N and neoprene.
2. Fibres: Fibres represent a class of polymers which are thread-like and can be woven into fabrics in a number of ways. These are widely used for making clothes, nets, ropes, gauzes etc. Fibres possess high tensile strength because the chains possess strong intermolecular forces such as hydrogen bonding. These forces are also responsible for close packing of the chains. As a result, the fibres are crystalline in nature and have aJso sharp melting points. A few common polymers belonging to this class are nylon – 66, terylene and polyacrylonitrile etc.
3. Thermoplastics: These are linear polymers and have weak van der Waals forces acting in the various chains and are intermediate of the forces present in the elastomers and in the fibres. When heated, they melt and form a fluid which sets into a hard mass on cooling, Thus, they can be cast into different shapes by using suitable moulds. A few common examples are polyethene and polystyrene polyvinyls etc. These can be used for making toys, buckets, telephone apparatus, television cabinets etc.
4. Thermosetting plastics: These are normally semifluid substances with low molecular masses. When heated, they become hard and infusible due to the cross-linking between the polymer chains. As a result, they also become three dimensional in nature. They do not melt when heated. A few common thermosetting polymers are bakelite, melamine-formaldehyde, urea-formaldehyde and polyurethane etc.
15.8. How can you differentiate between addition and condensation polymerisatiop?
Ans: In addition polymerization, the molecules of the same or different monomers simply add on to one another leading to the formation of a macromolecules without elimination of small molecules like H2O, NH3 etc. Addition polymerization generally occurs among molecules containing double and triple bonds. For example, formation of polythene from ethene and neoprene from chloroprene, etc. In condensation polymerisation, two or more bifunctional trifimctional molecules undergo a series of independent condensation reactions usually with the elimination of simple molecules like water, alcohol, ammonia, carbon dioxide and hydrogen chloride to form a macromolecule. For example, nylon-6,6 is a condensation polymer of hexamethylenediamine and adipic acid formed by elimination of water molecules.
15.9. Explain the term copolymerisation and give two examples.
Ans: When two or more different monomers are allowed to polymerise together the product formed is called a copolymer, and the process in called copolymerisation. Example, Buna-S and Buna-N. Buna- S is a copolymer of 1, 3- butadiene and styrene while Buna-N is a copolymer of 1,3-butadiene and acrylonitrile.
15.10. Write the free radical mechanism for the polymerisation of ethene.
Ans:

15.11. Define thermoplastics and thermo setting polymers with two examples of each
Ans: Thermoplastics polymers are linear polymer which can be repeatedly melted and moulded again and again on heating without any change in chemical composition and mechanical strength. Examples are polythene and polypropylene.
Thermosetting polymers, on the other hand, are permanently setting polymers. Once on heating in a mould, they get hardened and set, and then cannot be softened again. This hardening on heating is due to cross- linking between different polymeric chains to give a three dimensional network solid. Examples are bakelite, melamine-foimaldehyde polymer etc.
15.12. Write the monomers used for gettingThe following polymers:
(i) Polyvinylchloride
(ii) Teflon (iii) Bakelite
Ans:
15.13. Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Ans:
15.14. How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Ans: Natural rubber is cis-polyisoprene and is obtained by 1, 4-polymerization of isoprene units. In this polymer, double bonds are located between C2 and C3 of each isoprene unit. These cis-double bonds do not allow the polymer chains to come closer for effective interactions and hence intermolecular forces are quite weak. As a result, natural rubber, i.e., cis-polyisoprene has a randomly coiled structure not the linear one and hence show elasticity.
15.15. Discuss the main purpose of vulcanisation of rubber.
Ans: Natural rubber has the following disadvantages:
(a) It is soft and sticky and becomes even more so at high temperatures and brittle at low temperatures. Therefore, rubber is generally used in a narrow temperature range (283-335 K) where its elasticity is maintained.
(b)It has large water absorption capacity, has low tensile strength and low resistance to abrasion.
(c)It is not resistant to the action of organic solvents.
(d)It is easily attacked by oxygen and other oxidising agents. .
To improve all these properties, natural rubber is vulcanised by heating it with about 5% sulphur at 373-415 K. The vulcanized rubber thus obtained has excellent elasticity over a larger range of temperature, has low water absorption tendency and is resistant to the action of organic solvents and oxidising agents.
15.16. What are the monomeric repeating units of Nylon-6 and Nylon 6,6?
Ans:
15.17. Write the names and structures of the monomers of the following polymers:
(i) Buna-S (ii) Buna-N (iii) Dacron (iv) Neoprene
Ans:
15.18. Identify the monomer in the following polymeric structures:
Ans:
15.19. How is dacron obtained from ethylene glycol and terephthalic acid?
Ans: Dacron is obtained by condensation polymerization of ethylene glycol and terephthalic acid with the elimination of water molecules. The reaction is carried out at 420 – 460 K in presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide.
15.20. What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Ans: Polymers which disintegrate by themselves over a period of time due to environment degradation by bacteria, etc., are called biodegradable polymers. Example is PHBV, i. e., Poly-β-Hydroxybutyrate-co-β- Hydroxyvalerate.
Class 12 Chemistry Chapter 16 Chemistry in Everyday Life:
| Section Name | Topic Name |
| 16 | Chemistry in Everyday Life |
| 16.1 | Drugs and their Classification |
| 16.2 | Drug-Target Interaction |
| 16.3 | Therapeutic Action of Different Classes of Drugs |
| 16.4 | Chemicals in Food |
| 16.5 | Cleansing Agents |
NCERT INTEXT QUESTIONS
16.1. Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor. Why?
Ans: Most of drugs taken in doses higher than recommended may produce harmful effects and act as poison and cause even death. Therefore, a doctor must always be consulted before taking the drug.
16.2. “Ranitidine is an antacid” With reference to which classification, has this statement been given?
Ans: Ranitidine is labelled as antacid since it is quite effective in neutralising the excess of acidity in the stomach. It is sold in the market under the trade name Zintac.
16.3. Why do we require artificial sweetening agents?
Ans: To reduce calorie intake and to protect teeth from decaying, we need artificial sweeteners.
16.4. Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structures of these compounds are given below:
(i) (C15H31COO)3C3H5-Glyceryl palmitate
(ii) (C17H32COO)3C3H5-Glyceryl oleate
Ans:

16.5. Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic part in the molecule. Identify the functional group (s) present in the molecule.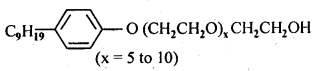
Ans: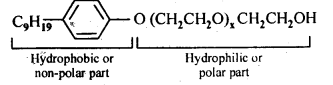
Functional groups present in the detergent molecule are:
(i)ether
(ii)1°alcoholic group
NCERT EXERCISES
16.1. Why do we need to classify drugs in different ways?
Ans: Drugs are classified in following different ways:
(a) Based on pharmacological effect.
(b) Based on action on a particular biochemical process.
(c) Based on chemical structure.
(d) Based on molecular targets.
Each classification has its own usefulness.
(а) Classification based on pharmacological effect is useful for doctors because it provides them the whole range of drugs available for the treatment of a particular disease.
(b) Classification based on action on a particular biochemical proc*ess is useful for choosing the correct compound for designing the synthesis of a desired drug.
(c) Classification based on chemical structure helps us to design the synthesis of a number of structurally similar compounds having different substituents and then choosing the drug having least toxicity.
(d) Classification on the basis of molecular targets is useful for medical chemists so that they can design a drug which is most effective for a particular receptor site.
16.2. Explain the following as used in medicinal chemistry
(a) Lead compounds
(b) Target molecules or drug targets.
Ans:
(a) Lead compounds are the compounds which are effective in different drugs. They have specific chemical formulas and may be extracted either from natural sources (plants and animals) or may be synthesised in the laboratory.
(b) Target molecules or drug targets. An enzyme (E) functions by combining with the reactant (called substrate) denoted as ‘S’ to form an activated complex known as enzyme-substrate complex (E-S). The complex dissociates to form product and releases the enzyme for carrying out further activity.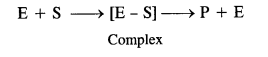
16.3. Name the macro molecules that are chosen as drug targets.
Ans: Proteins, carbohydrates, lipids and nucleic acids are chosen as drug targets.
16.4. Why the medicines should not be taken without consulting doctors?
Ans: No doubt medicines are panacea for most of the body ailments. But their wrong choice and overdose can cause havoc and may even prove to be fatal. Therefore, it is of utmost importance that the medicines should not be given without consulting doctors.
16.5. Define the term chemotherapy.
Ans: It is the branch of chemistry that deals with the treatment of diseases by using chemicals as medicines.
16.6. Which forces are involved in holding the drugs to the active site of enzymes?
Ans: The following forces are involved in holding the drugs to the active site of enzymes:
(a) Hydrogen bonding
(b) Ionic bonding
(c) Dipole-dipole interactions
(d) van der Waals interactions
16.7. Antacids and antiallergic drugs interfere with the function of histamines but do not interfere with the function of each other. Explain.
Ans: They donot interfere with the functioning of each other because they work on different receptors in the body.Histamine stimulates the secretion of pepsin and hydrochloric acid in the stomach. The drug cimetidine (antacid) was designed to prevent the interaction of histamine with the receptors present in the stomach wall. This resulted in release of lesser amount of acid. Antacid and antiallergic drugs work on different receptors.
16.8. Low level of noradrenaline is the cause of depression. What type of drugs are needed to cure this problem? Name two drugs.
Ans: In case of low level of neurotransmitter, . noradrenaline, tranquilizer (antidepressant) drugs are required because low levels of noradrenaline leads to depression. These drugs inhibit the enzymes which catalyse the degradation of noradrenaline. If the enzyme is inhibited, noradrenaline is slowly metabolized and can activate its receptor for longer periods of time thereby reducing depression. Two important drugs are iproniazid and phenylzine.
16.9. What is meant by the term broad spectrum antibiotics? Explain.
Ans: Broad spectrum antibiotics are effective against several different types or wide range of harmful bacteria. For example, tetracycline, chloramphenicol and of loxacin. Chloramphenicol can be used in case of typhoid, dysentry, acute fever, urinary infections, meningitis and pneumonia.
16.10. How do antiseptics differ from disinfectants ? Give one example of each.
Ans: Many times, the same substance can act as an antiseptic as well as disinfectant by changing the concentration of the solution used. For example, a 0.2 per cent solution of phenol acts as an antiseptic and its 1 percent solution is a disinfectant. Chlorine is used in India for making water fit for drinking at a concentration of 0.2 to 0.4 ppm (parts per million). Low concentration of sulphur dioxide is used for sterilizing squashes for preservation. A few points of distinction between antiseptics and disinfectants are listed.
| Antiseptics | Disinfectants |
| 1. Can kill or prevent the growth of micro-organisms. | 1.Can kill micro-organisms. |
| 2. Do not harm the living tissues. Therefore, these can be applied to the skin. | 2. Toxic to the living tissues. Therefore, these cannot be applied to the skin. |
| 3. These are used for the dressing of wounds, ulcers and in the treatment of diseased skin. | 3. These are used for disinfecting floors, toilets, drains, instruments etc. |
16.11. Why are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide?
Ans: If excess of NaHCO3 or Mg(OH)2 or Al(OH)3 is used, it makes the stomach alkaline and thus triggers the release of even more HCl which may cause ulcer in the stomach. In contrast, cimetidine and ranitidine prevent the interaction of histamine with the receptor cells in the stomach wall and thus release of HCl will be less as histamine stimulates the secretion of acid.
16.12. Name a substance which can be used as an antiseptic as well as disinfectant.
Ans: 0.2% solution of phenol acts as antiseptic while 1% solution acts as a disinfectant.
16.13. What are the main constituents of dettol?
Ans: Chloroxylenol .and α-terpineol in a suitable solvent.
16.14. What is tincture of iodine? What is its use?
Ans: 2-3% solution of iodine in alcohol and water is called tincture of iodine. It is a powerful antiseptic. It is applied on wounds.
16.15. What are food preservatives?
Ans: Preservation has a major role in the food products. Chemically preserved squashes and crushes can be kept for a fairly long time even after opening the seal of bottle.
A preservative may be defined as the substance which is capable of inhibiting or arresting the process of fermentation, acidification or any other decomposition of food. Salting i.e. addition of table salt is a well known method for food preservation and was applied in ancient times for preserving raw mangoes, tamarind, meat, fish etc. Sugar syrup can also act as a preservative. Vinegar is a useful preservative for pickles. Apart from these, sulphur dioxide and benzoic acid can be employed for the preservation of food. The major source of sulphur dioxide is potassium metabisulphite (K2S2O5). It is fairly stable in neutral and alkaline medium but gets decomposed by weak acids such as carbonic, citric, tartane and malic acids. Benzoic acid is used either as such or in the form of sodium benzoate. However, sulphur dioxide has a better preservative action than sodium benzoate against bacteria and moulds. It also retards the development of yeast in juice but fails to arrest their multiplication once the number has reached a high value. Sorne salts of sorbic acid and propionic acid are also being used these days for the preservation of the food.
The use of preservatives must be properly controlled as their indiscriminate use is likely to be harmful. The preservative should not be injurious to health and should be also non-irritant.
16.16. Why is the use of aspartame limited to cold foods and drinks?
Ans: This is because it decomposes at baking or cooking temperatures and hence can be used only in cold foods and drinks as an artificial sweetener
16.17. What are artificial sweetening agents? Give two examples.
Ans: Artificial sweeteners are chemical substances which are sweet in taste but do not add any calories to our body. They are excreted as such through urine. For example, saccharin, aspartame, alitame etc.
16.18. Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Ans: Saccharine, aspartame or alitame may be used in the preparation of sweets for a diabetic patient.
16.19. What problem arises in using alitame as artificial sweetener?
Ans: Alitame is a high potency artificial sweetener.Therefore, it is difficult to control the sweetness of the food to which it is added.
16.20. How are synthetic detergents better than soaps?
Ans: They can be used in hard water as well as in acidic solution. The reason being that sulphonic acids and their calcium and magnesium salts are soluble in water thus they do not form curdy white precipitate with hard water but the fatty acids and their calcium and magnesium salts of soaps are insoluble. Detergents also works in slightly acidic solution due to formation of soluble alkyl hydrogen sulphates. Soaps react with acidic solution to form insoluble fatty acids.
16.21. Explain the following terms with suitable examples:
(i) cationic detergents (ii) anionic detergents and (iii) non-ionic detergents
Ans: (i) Cationic detergents: These are quaternary ammonium salts, chlorides, acetates, bromides etc containing one or more long chain alkyl groups. For example, cetyltrimethyl ammonium chloride.
(ii) Anionic detergents are called so because a large part of their molecules are anions. ‘These are of two types:
(a) Sodium alkyl sulphates: For example, sodium lauryl sulphate, C11H23CH2OSO3 Na+.
(b) Sodium alkylbenzenesulphonates.Vor example, sodium 4-(l-dodecyl) benzenesu Iphphonate (SDS).
(iii) Neutral or non-ionic detergents: These are esters of high molecular mass alcohols with fatty acids. These can also be obtained by treatment of long chain alcohols by with excess of ethylene oxide in presence of a base. For example, polyethylene glycol stearate,CH3(CH2)16COO (CH2CH2O)11 CH2CH2OH Polyethylene glycol stearate.
16.22. What are biodegradable and non-biodegradable detergents? Give one example of each.
Ans: Detergents having straight chain hydrocarbons are easily degraded (or decomposed) by microorganisms and hence are called biodegradable detergents while detergents containing branched hydrocarbon chains are not easily degraded by the microorganisms find hence are called non-biodegradable detergents. Consequently, non-biodegradable detergents accumulate in rivers and water ways thereby causing severe water pollution. Examples of biodegradable detergents are sodium lauryl sulphate, sodium 4-(-l-dodecyl) benzenesulphonate and sodium 4-(2-dodecyl) benzenesulphonate.
Examples of non-biodegradable detergents is sodium 4-(1, 3,5,7 – tetramethyloctyl) benzene sulphonate.
16.23. Why do soaps not work in hard water? (C.B.S.E. Outside Delhi 2009, 2011)
Ans: Soaps are water soluble sodium or potassium salts of higher fatty acids like palmitic acid (C15H31COOH), oleic acid (C17H33COOH) and stearic acid (C17H35COOH). Hard water contains certain calcium and magnesium salts which combine with soaps to form corresponding magnesium compounds. These being insoluble, get separated as curdy white precipitates resulting in wastage of soap.
16.24. Can you use soaps and synthetic detergents to check the hardness of water?
Ans: Soaps get precipitated as insoluble calcium and magnesium soaps in hard water but detergents do not. Therefore, soaps but not synthetic detergents can be used to check the hardness of water.
16.25. Explain the cleansing action of soaps.
Ans: Cleansing action of soaps : Soaps contain two parts, a large hydrocarbon which is a hydrophobic (water repelling) and a negative charged head, which is hydrophillic (water attracting). In solution water molecules being polar in nature, surround the ions & not the organic part of the molecule. When a soap is dissolved in water the molecules gather together as clusters, called micelles. The tails stick inwards & the head outwards. The hydrocarbon tail attaches itself to oily dirt. When water is agitated, the oily dirt tends to lift off from the dirty surface & dissociates into fragments. The solution now contains small globules of oil surrounded detergent molecules. The negatively charged heads present in water prevent the small globules from coming together and form aggregates. Thus the oily dirt is removed from the object.
16.26. If water contains dissolved calcium hydrogencarbonate, out of soaps and synthetic detergents, which one will you use for cleaning clothes?
Ans: Calcium hydrogencarbonate makes water hard. Therefore, soap cannot be used because it gets precipitated in hard water. On the other hand, a synthetic detergent does not precipitate in hard water because its calcium salt is also soluble in water. Therefore, synthetic detergents can be used for cleaning clothes in hard water.
16.27. Label the hydrophilic and hydrophobic parts in the following compounds.
(i)cCH3(CH2)10CH2OSO3 –Na+
(ii) CH3(CH2)15 -N+(CH3)3Br–
(iii) CH3(CH2)16C00(CH2CH2O)11CH2CH2OH
Ans:


Leave a Reply